Subfamily: Alpharhabdovirinae
Genus: Hapavirus
Distinguishing features
Viruses assigned to the genus Hapavirus form a distinct monophyletic group based on well-supported Maximum Likelihood or Maximum Clade Credibility trees inferred from complete L sequences. Hapaviruses have been isolated primarily from birds and culicine mosquitoes. The hapavirus clade is part of a larger phylogenetic group of arthropod-borne rhabdoviruses with large and complex genomes that also includes ephemeroviruses, tibroviruses and curioviruses. Most hapaviruses contain i) multiple genes between the P gene and M gene encoding proteins that share low levels of sequence homology and appear to have arisen by gene duplication; and ii) a gene between the G and L genes encoding a small class 1a viroporin-like protein.
Virion
Morphology
Virion morphology in ultrathin sections of infected cells has been described for several hapaviruses. For Flanders virus (FLAV; species Hapavirus flanders), Mossuril virus (MOSV; species Hapavirus mossuril) and Manitoba virus (MANV; species Hapavirus manitoba), long bullet-shaped virions (about 200–300 nm × 45–75 nm) have been observed budding at the plasma membrane and/or in association with intracytoplasmic vacuoles (Karabatsos et al., 1973, Boyd and Whitaker-Dowling 1988, Artsob et al., 1991). Wongabel virus (WONV; species Hapavirus wongabel) virions have also been observed budding from the plasma membrane and endoplasmic reticula but are shorter and more cone-shaped (160–180 nm × 80–90 nm). Bullet-shaped morphology (60 nm × 175 nm) has also been observed for Bangoran virus (BGNV; species Hapavirus bangoran) (El Mekki et al., 1981).Variations in virion morphology may be due in part to differences in conditions used for fixation and staining of tissues (Gubala et al., 2008).
Nucleic acid
Hapavirus genomes consist of a single molecule of negative-sense, single-stranded RNA and range from approximately 12.4–15.8 kb (Boyd and Whitaker-Dowling 1988, Gubala et al., 2008, Gubala et al., 2010, Allison et al., 2014, Walker et al., 2015).
Proteins
The N, P, M, G and L share sequence homology and/or structural characteristics with the cognate proteins of other rhabdoviruses. Few other proteins that are encoded in hapavirus genomes have yet been identified in infected cells. P-M intergenic region proteins (PMIPs) range from 142 to 194 amino acids (16.5–22.3 kDa) and generally share very low but detectable sequence homology with PMIPs in the same virus and in other closely related hapaviruses (Walker et al., 2015). Of the many hapavirus PMIPs, only WONV U3 has been shown to be expressed and characterised functionally, binding to a component of the SWI/SNF chromatin remodelling complex and regulating immune response gene expression (Joubert et al., 2015). Small class 1a viroporin-like proteins encoded in all hapaviruses (except Marco virus (MCOV; species Hapavirus marco) range from 102 to 153 amino acids (11.5–17.6 kDa) and feature an N-terminal domain containing large hydrophobic residues, a predicted hydrophobic transmembrane domain and a C-terminal domain that is rich in basic residues (Walker et al., 2015). The 568-amino-acid Ngaingan virus (NGAV; species Hapavirus ngaingan) GNS protein is predicted to be a class I transmembrane glycoprotein (unglycosylated mass 65.0 kDa) that shares extensive sequence homology with NGAV G and G of other rhabdoviruses; similar GNS proteins also occur in all ephemeroviruses but phylogenetic inferences suggest that the NGAV GNS protein has evolved either independently or by genetic recombination (Gubala et al., 2010, Walker et al., 2015). The 459 amino acid MCOV U1 protein is also predicted to be a second class I transmembrane protein (unglycosylated mass 52.5 kDa) but it lacks cysteine residues that could form disulphide bonds and appears to be unrelated to either MCOV G or NGAV GNS (Walker et al., 2015). The many other putative hapavirus accessory proteins are predicted to have largely unremarkable structural characteristics.
Genome organisation and replication
Hapavirus genomes include five genes (N, P, M, G and L) encoding the structural proteins and multiple additional long ORFs (Figure 1 Hapavirus). The genomes vary considerably in the number and locations of accessory genes but most hapavirus genomes include: i) multiple genes between the P gene and M gene encoding proteins that share low levels of sequence identity and appear to have arisen by gene duplication; and ii) a gene between the G and L genes encoding a small class 1a viroporin-like protein (Walker et al., 2011, Allison et al., 2014, Walker et al., 2015). Several other hapavirus genes appear to have arisen by gene duplication: in NGAV, the G gene and the GNS gene immediately following encode glycoproteins that share extensive sequence homology; and in Joinjakaka virus (JOIV; species Hapavirus joinjakaka), the U2 and U3 genes, also located between the G gene and L gene, share significant sequence homology. In WONV, Ord River virus (ORV; species Hapavirus ord) and Parry Creek virus (PCV; species Hapavirus parry), alternative long ORFs in the N genes (Nx or U4) overlap the end of the N ORF and encode homologous proteins; similarly, FLAV and Hart Park virus (HPV; species Hapavirus hartpark) encode homologous proteins in alternative ORFs (also designated Nx) near the end of the N gene.
Many of the additional hapavirus long ORFs occur in novel independent transcriptional units including conserved transcription initiation and transcription termination sequences. Others are located as alternative, overlapping or consecutive ORFs within the structural protein genes or within the novel transcriptional units. Although yet to be tested experimentally, hapaviruses therefore appear to employ various non-canonical strategies to express proteins encoded in these long ORFs. The likely mechanisms include: i) leaky ribosomal scanning; ii) a stop-start mechanism involving a ‘termination upstream ribosome-binding site’ (TURBS); and iii) ribosomal frame shifts featuring a ‘slippery’ sequence followed by a predicted pseudoknot structure (Walker et al., 2015).
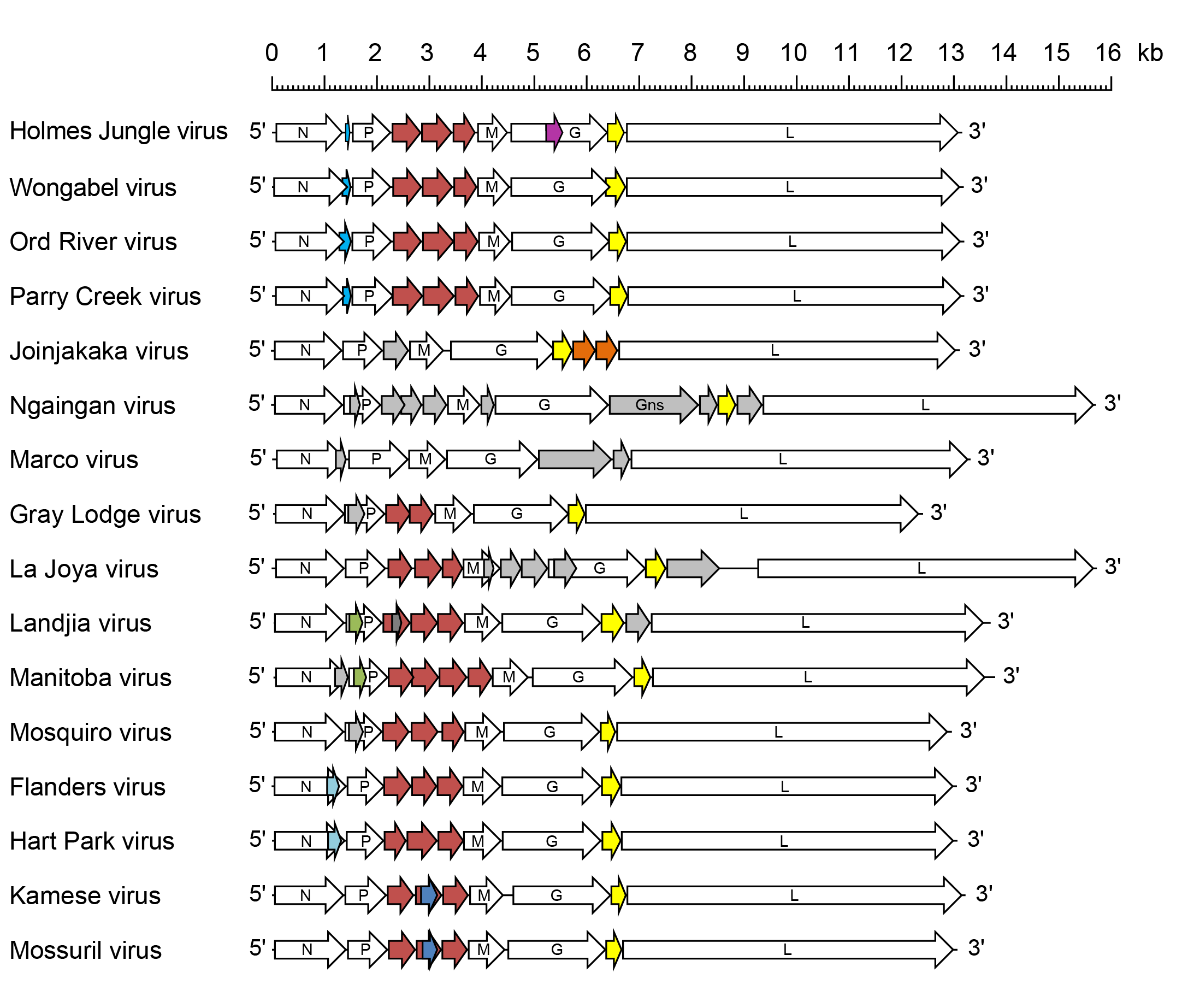 |
| Figure 1 Hapavirus. Schematic representation of hapavirus genomes shown in reverse (positive-sense) polarity. N, P, M, G and L represent ORFs encoding the structural proteins. ORFs are indicated as block arrows. P-M intergenic region protein (PMIP) ORFs are coloured in red; class 1a viroporin-like protein ORFs are coloured in yellow; other colours indicate ORFs encoding homologous proteins or alternative ORFs that occur in some genes; only ORFs (≥180 nt) that appear likely to be expressed are shown. |
Biology
Hapaviruses have been isolated primarily from culicine mosquitoes and passerine birds. HPV and FLAV circulate in mosquito-bird transmission cycles in western and eastern regions of the USA, respectively (Allison et al., 2014). Landjia virus (LJAV; species Hapavirus landjia) was isolated from a passerine bird (Riparia paludicola) in Africa; other hapaviruses have been isolated from culicine mosquitoes in Africa (MOSV, BGNV, Kamese virus [KAMV; species Hapavirus kamese]), North America (MANV, Gray Lodge virus [GLOV; species Hapavirus graylodge]), Central America (La Joya virus [LJV; species Hapavirus lajoya]), South America (Mosqueiro virus [MQOV; species Hapavirus mosqueiro]), South-East Asia (Porton virus; species Hapavirus porton), Papua New Guinea (JOIV) or Australia (ORV, PCV). WONV and NGAV were each isolated from biting midges (Culicoides spp.) in Australia (Gubala et al., 2008, Gubala et al., 2010). There is evidence of WONV antibodies in sea birds (Humphrey-Smith et al., 1991) and NGAV antibodies in marsupials (wallabies and wallaroos) (Gubala et al., 2010). MCOV was isolated from reptiles (lizards) in South America (Causey et al., 1966).
Antigenicity
Several members of the genus Hapavirus (HPV, FLAV, MQOV, MOSV, KAMV) cross-react strongly in complement-fixation and indirect immunofluorescence tests and have been assigned to the Hart Park serogroup of rhabdoviruses (Tesh et al., 1983, Calisher et al., 1989). Several of the viruses in this serogroup also cross-react weakly in neutralisation tests.
Species demarcation criteria
Viruses assigned to different species within the genus Hapavirus display several of the following characteristics: A) minimum amino acid sequence divergence of 5% in the N proteins; B) minimum amino acid sequence divergence of 10% in the L proteins; C) minimum amino acid sequence divergence of 15% the G proteins; D) significant differences in genome organisation as evidenced by numbers and locations of ORFs; E) can be distinguished in virus neutralisation tests; and F) occupy different ecological niches as evidenced by differences in hosts and/or arthropod vectors.

