Subfamily: Alpharhabdovirinae
Genus: Curiovirus
Distinguishing features
Viruses assigned to the genus Curiovirus form a distinct monophyletic group based on well-supported Maximum Likelihood or Maximum Clade Credibility trees inferred from complete L sequences. Curioviruses have been isolated from haematophagous insects (biting midges, sandflies and mosquitoes) collected in South America. The curiovirus clade is part of a larger phylogenetic group of insect-transmitted rhabdoviruses with large and complex genomes that also includes ephemeroviruses, tibroviruses and hapaviruses. Curiovirus genomes feature multiple accessory genes including i) one or more genes between the M gene and G gene encoding small proteins of unknown function; and ii) one or more genes between the G gene and L gene, including a gene encoding a small class 1a viroporin-like protein.
Virion
Morphology
Curionopolis virus (CURV; species Curiovirus curionopolis) and Itacaiunas virus (ITAV; species Curiovirus itacaiunas) virions with bullet-shaped morphology (approximately 180 nm × 80 nm) have been observed budding from plasma membranes in ultrathin sections of mouse neuronal cells (Diniz et al., 2006, Diniz et al., 2008).
Nucleic acid
Curiovirus genomes consist of a single molecule of negative-sense, single-stranded RNA and range from 12.6–13.6 kb (Medeiros et al., 2014, Walker et al., 2015).
Proteins
The N, P, M, G and L share sequence homology and/or structural characteristics with the cognate proteins of other rhabdoviruses. Other proteins encoded in curiovirus genomes have not yet been identified in infected cells (Walker et al., 2015). Putative curiovirus U1 proteins range from 82 to 87 amino acids (9.3–9.9 kDa). The CURV, Iriri virus (IRIRV; species Curiovirus iriri) and Rochambeau virus (RBUV; species Curiovirus rochambeau) U1 proteins are orthologous (pairwise sequence identity = 65.9–69.5 %); the ITAV U1 protein also appears to be orthologous but is somewhat more divergent. Putative curiovirus U1x proteins range from 65 to 74 amino acids (7.5–8.6 kDa). The CURV, IRIRV and RBUV U1x proteins are orthologous (pairwise sequence identity = 43.7–50.0 %); ITAV lacks the U1x protein. Putative curiovirus U2 proteins range from 91 to 105 amino acids (10.0–11.7 kDa). The CURV and IRIRV U2 proteins are orthologous (pairwise sequence identity = 45.8 %); the RBUV U2 protein displays low level sequence homology with other curiovirus U2 proteins and the U1 protein of ITAV which lacks a U2 protein. The putative RBUV U3 protein (134 amino acids; 15.2 kDa) is unique. Putative CURV U3, IRIRV U3, RBUV U4 and ITAV U2 proteins are class 1a viroporin-like proteins featuring an N-terminal domain containing large hydrophobic residues, a central transmembrane domain and a highly basic C-terminal domain. Putative CURV U3x, IRIRV U3x and RBUV U4x proteins are each 115 amino acids (13.3–13.6 kDa) and are orthologous (pairwise sequence homology = 47.8–56.5%).
Genome organisation and replication
Curiovirus genomes include five genes (N, P, M, G and L) encoding the structural proteins and multiple additional long ORFs (Medeiros et al., 2014, Walker et al., 2015). The genomes of all curioviruses include: i) one gene (U1) between the M gene and G gene encoding a small protein of unknown function; and ii) one gene (U2, U3 or U4 depending on the virus) between the G gene and L gene encoding a small class 1a viroporin-like protein (Figure 1 Curiovirus). In CURV, IRIRV and RBUV genomes, there are three long ORFs between the M gene and the G gene: U1 and U1x are overlapping ORFs in one transcriptional unit (gene); ORF U2 lies in an independent gene immediately following the U1/U1x gene. The ITAV genome contains ORF U1 but lacks orthologues of the U1x and U2 ORFs. In CURV and IRIRV genomes, there are two consecutive ORFs (U3 and U3x) in a single transcriptional unit between the G gene and L gene. The CURV and IRIRV U3 ORFs encode class 1a viroporin-like proteins. Corresponding consecutive ORFs (U4 and U4x) also occur as within a single transcriptional unit in the RBUV genome but it is preceded by another gene (RBUV U3) that is unique. The ITAV genome has a single ORF (ITAV U2) in an independent transcriptional unit between the G gene and L gene; it encodes a class 1a viroporin-like protein.
Curioviruses appear to employ non-canonical strategies to express overlapping or consecutive ORFs within single transcriptional units (Walker et al., 2015). The CURV, IRIRV and RBUV overlapping ORFs U1 and U1x feature a ‘slippery sequence’ and potential ribosomal frame-shift site in the overlap region. Consecutive ORFs within single transcriptional units in the CURV and IRIRV genomes (U3/U3x) and in the RBUV genome (U4/U4x) feature a ‘termination upstream ribosome-binding site’ (TURBS) that would allow a stop-start mechanism of translation.
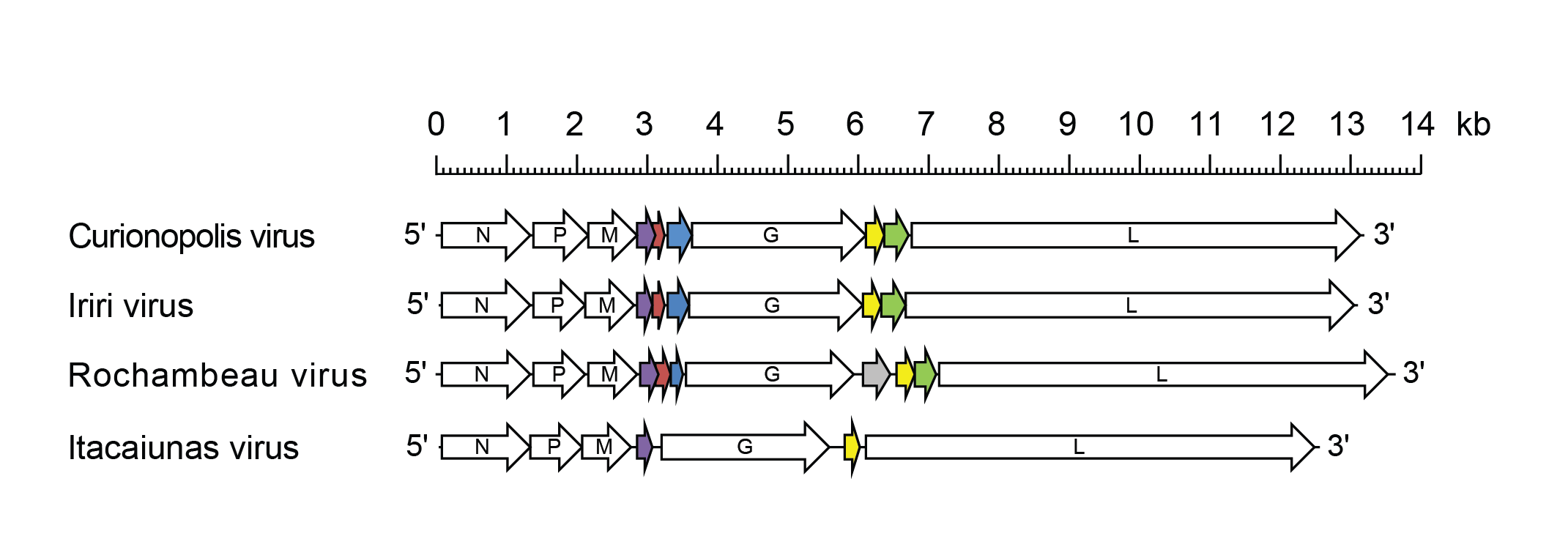 |
| Figure 1 Curiovirus. Schematic representation of curiovirus genomes shown in reverse (positive-sense) polarity. N, P, M, G and L represent ORFs encoding the structural proteins. The U1 (purple), U1x (red brown) and U2 (blue) ORFs encode orthologous sets of accessory proteins. The U3 ORFs of Curionopolis virus and Iriri virus, the U4 ORF of Rochambeau virus and the U2 ORF of Itacaiunas virus each encode viroporin-like proteins (yellow). The U3x ORFs of Curionopolis virus and Iriri virus, and the Rochambeau virus U4x ORF also encode orthologous sets of accessory proteins (green). [Note that the Itacaiunas virus U1 protein also shares a low level of homology with the Rochambeau virus U2 protein]. The U3 ORF of Rochambeau virus encodes unique protein of unknown function (grey). |
Biology
Curioviruses have been isolated from haematophagous insects (biting midges, sandflies and mosquitoes) from tropical regions of South America. CURV and ITAV were isolated from biting midges (Culicoides sp.) and IRIRV was isolated from sandflies (Lutzomyia sp.), each collected from Para State, Brazil (Travassos da Rosa et al., 1998, Diniz et al., 2006). Neutralising antibodies to CURV have been reported in a tufted capuchin (Cebus paella) and in a South American coati (Nasua nasua) (Diniz et al., 2006). RBUV was isolated from mosquitoes (Coquillettidia albicosta) collected in nearby Paramana in French Guiana (Digoutte 1974, Karabatsos 1985).
Antigenicity
CURV and ITAV do not cross-react with each other in complement-fixation, indirect immunofluorescence or neutralisation tests (Diniz et al., 2006). RBUV has been reported to cross-react weakly with rabies virus and Sandjimba virus (related, unclassified rhabdovirus) in indirect immunofluorescence tests (Calisher et al., 1989).
Species demarcation criteria
Viruses assigned to different species within the genus Curiovirus have several of the following characteristics: A) minimum amino acid sequence divergence of 5% in the N proteins; B) minimum amino acid sequence divergence of 10% in the L proteins; C) minimum amino acid sequence divergence of 15% in the G proteins; D) significant differences in genome organisation as evidenced by numbers and locations of ORFs; E) can be distinguished in virus neutralisation tests; and F) occupy different ecological niches as evidenced by differences in hosts and/or arthropod vectors.

