Family: Spinareoviridae
Genus: Coltivirus
Distinguishing features
The coltivirus genome consists of 12 segments of dsRNA. During replication, virions are found in the cell cytoplasm, associated with granular matrices (viral inclusion bodies: VIB), arrays of filaments or tubules, and fine kinky threads. Immunofluorescent staining reveals nucleolar fluorescence. Viruses are transmitted to vertebrate hosts by tick vectors.
Virion
Morphology
Coltivirus particles are 60–80 nm in diameter having two concentric capsid shells with a core that is about 50 nm in diameter. Electron microscopic studies, using negative-staining, have shown that particles have a surface with a capsomeric structure and icosahedral symmetry (Figure 1 Coltivirus). Particles are found intimately associated with filamentous structures and granular matrices in the cytoplasm. Most virus particles are non-enveloped, but a few acquire an envelope structure during their passage through the endoplasmic reticulum.
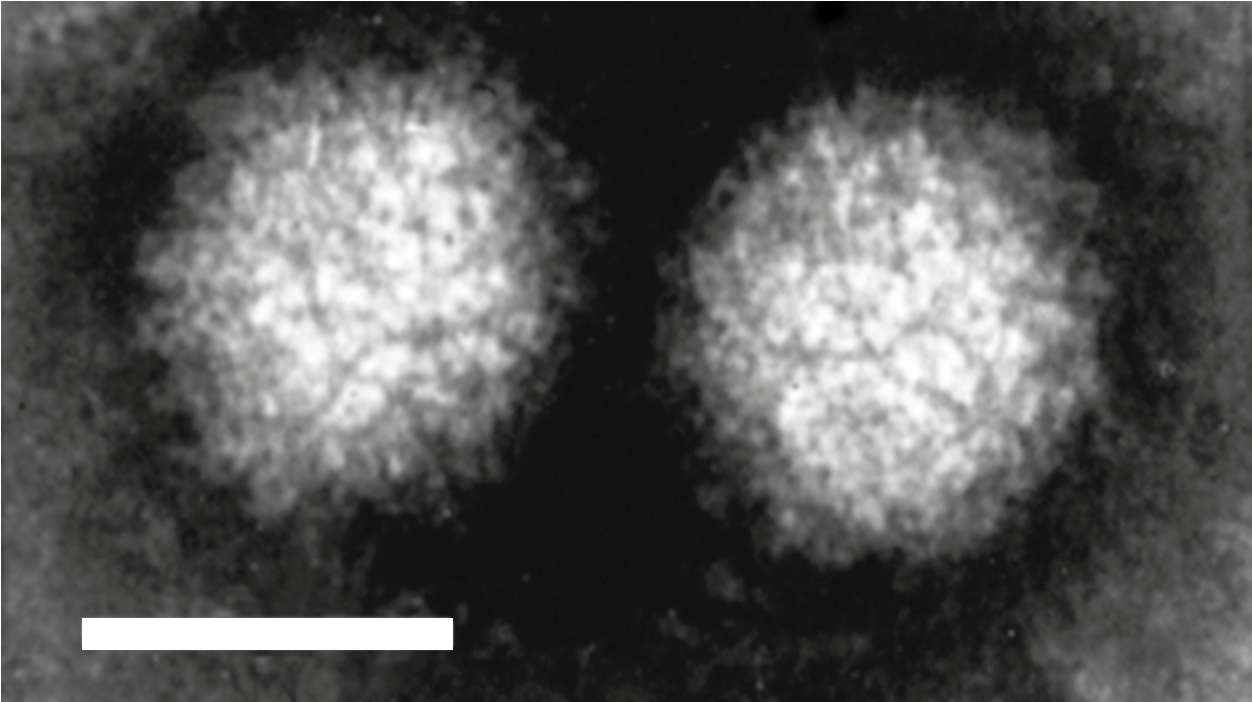 |
| Figure 1 Coltivirus Negative-contrast electron micrograph of particles of Colorado tick fever virus (CTFV) (courtesy of F. A. Murphy). The bar represents 50 nm. |
Physicochemical and physical properties
The buoyant density of virions in CsCl is 1.38 g cm−3. Virions are stable between pH 7 and 8, but lose infectivity at pH 3.0. At 4 °C, the virion is stable for long periods when stored in presence of 50% fetal calf serum in 0.2 M Tris-HCl pH 7.8. Heating to 55 °C considerably decreases infectivity. Coltiviruses are fairly stable upon treatment with non-ionic detergents, sodium lauroyl sarcosine, or freon but infectivity is abolished by treatment with sodium deoxycholate or sodium dodecyl sulfate. Moderate ultrasonic oscillation treatment does not destroy infectivity and can be used in virion purification. Virions can be stored for long periods at −80 °C, and infectivity is further protected by addition of 50% fetal calf serum.
Nucleic acid
The genome consists of 12 dsRNA segments that are numbered in order of decreasing length, or increasing electrophoretic mobility during agarose gel electrophoresis. The genome comprises approximately 29,000 bp, with individual segments ranging from 4,350 and 675 bp. The genomic RNAs of Colorado tick fever virus (CTFV) migrate in three length classes (Figure 2 Coltivirus) during 1% agarose gel electrophoresis (AGE): the long or L-segments (Seg1 to Seg4), the medium length or M-segments (Seg5 to Seg10) and the short or S-segments (Seg11 and Seg12). The dsRNA genome of Eyach virus (EYAV) has not yet been analyzed by AGE. The terminal 5′- and 3′-sequences of the coltivirus genome are conserved (Table 1 Coltivirus).
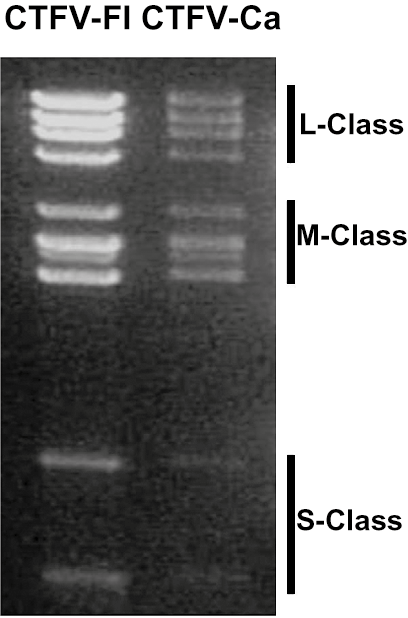 |
| Figure 2 Coltivirus Electrophoretic profiles of the genome segments of Colorado tick fever virus isolates Florio (CTFV-Fl) and California hare (CTFV-Ca) in 1% agarose gel. Segment length classes (L, large, M, medium and S small) are indicated to the right. |
RNA cross-hybridization analysis shows that CTFV isolates are relatively homogenous; distinct CTFV serotypes are also difficult to distinguish, although some sequence variation does occur, for example in Seg4 and Seg6. The overall nucleotide sequence identity between genome segments of different CTFV isolates, ranges between 90% and 100%. However, homologous segments of CTFV and EYAV are 55% to 86% identical. Seg7 from EYAV is partially homologous to Seg6 of CTFV, although the protein it encodes also has similarities to a sarcolemmal-associated protein from the European rabbit (Oryctolagus cuniculus (Linnaeus, 1758)), which is thought to be one of its major hosts. Coltivirus mRNAs are usually regarded as non-infectious. However fully functional and infectious virions have been recovered by the introduction of all 12 mRNAs into BSR cells (a clone of BHK-21 cells sensitive to rubella virus).
Table 1 Coltivirus Conserved coltivirus terminal sequences (positive-sense strand)
| Virus species | Virus name | 5′-end | 3′-end |
|---|---|---|---|
| Coltivirus dermacentoris | Colorado tick fever virus (CTFV) | 5′-G/CACAUUUUGU | UGCAGUG/C-3′ |
| Coltivirus ixodis | Eyach virus (EYAV) | 5′-GACAA/UUU | A/UUGC/UAGUC-3′ |
Proteins
See Table 2 Coltivirus.
Genome organization and replication
CTFV genome segments 1 to 8 and 10 to 12, each encode a single protein. However, Seg9 contains a leaky stop codon and two proteins are produced when it is translated in a cell-free system or in cells transfected with a plasmid containing the full length Seg9 cDNA. The shorter 38 kDa protein, (VP9ter) is the early-termination translation product, while the read-through protein (VP9rdt) is 67.3 kDa (Table 2 Coltivirus). The proteins encoded by EYAV have not been similarly characterized.
In cells infected by CTFV, granular matrices are produced which contain virus-like particles. These structures appear similar to VIBs produced during orbivirus infections. In addition, bundles of filaments (tubules), characterized by cross-striations, are found in the cytoplasm and, in some cases, in the nucleus of infected cells. These may also be comparable to the tubules found in orbivirus infected cells. There is no evidence for virion release prior to cell death and lysis, after which more than 90% of virions remain cell-associated. Immuno-fluorescence staining shows that viral proteins accumulate in the cytoplasm and could be detected from 12 hours post infection. Nucleolar fluorescence was also observed. Mosquito cells infected by EYAV develop syncitial foci. Electron microscopy of EYAV-infected mouse brain reveals similar intracellular structures to those observed in CTFV-infected cells.
The amino acid sequence (residues 370–490) of EYAV VP7 is 24% identical (50% similar) to the sarcolemmal-associated protein of the European rabbit. The corresponding region of the homologous VP6 protein of CTFV has no significant match with this rabbit protein. This suggests that a recombination event has occurred between viral and host RNAs leading to the formation of a novel genome segment with mixed ancestry. However, the level of similarity, suggests that this event occurred a very long time ago, possibly involving an ancestor of the European rabbit. Sequence analysis and phylogenetic studies suggest that EYAV is derived from an ancestral virus that was introduced into Europe during the migration of ancestors of lagomorphs (hares, rabbits) from North America through Asia. Lagomorph ancestors first appeared during the Eocene epoch (57.8–36.6 MYA) in what was then North America. They are thought to have first migrated into Asia during the Oligocene epoch (34–23 MYA) and by the high Miocene epoch (23–25 MYA) they were common in Europe (Attoui et al., 2002).
Table 2 Coltivirus Genome segments and protein products of Colorado tick fever virus
| Genome segment | bp | Protein nomenclature | Protein mass kDa* | Protein structure/function |
|---|---|---|---|---|
| Seg1 | 4350 | VP1 | 125 (163) | RNA-directed RNA polymerase (RdRP) |
| Seg2 | 3909 | VP2 | 117 (136) | Methyltransferase, cell-receptor |
| Seg3 | 3586 | VP3 | 113 (135) | Guanylyltransferase |
| Seg4 | 3157 | VP4 | 100 (112) | |
| Seg5 | 2432 | VP5 | 90 (84) | |
| Seg6 | 2141 | VP6 | 82 (78) | Nucleotide binding, NTPase |
| Seg7 | 2133 | VP7 | 75 (76) | RNA replication factors |
| Seg8 | 2029 | VP8 | 60 (74) | |
| Seg9 | 1884 | VP9ter/VP9rdt | 42 and 60 (38 and 67) | |
| Seg10 | 1880 | VP10 | 55 (69) | Kinase, helicase |
| Seg11 | 998 | VP11 | 34 (28.5) | |
| Seg12 | 675 | VP12 | 25 (20.4) | RNA replication factors |
* As measured from translation products or calculated from nucleotide sequences (in brackets).
Biology
Coltiviruses have been isolated from mammals of several species (including humans), as well as from ticks and mosquitoes which serve as arthropod vectors. Ticks that have been implicated include those of the species Dermacentor andersoni, D. occidentales, D. albipictus, D. parumapertus, Haemaphysalis leporispalustris, Otobius lagophilus, Ixodes sculptus, I. spinipalpis, I. ricinus and I. ventalloi.
Ticks can become infected with CTFV after ingestion of a blood meal from an infected vertebrate host. Both adult and nymphal ticks become persistently infected and provide an overwintering mechanism for the virus. CTFV is transmitted trans-stadially but not trans-ovarially. Some rodents have prolonged viraemia (more than 5 months) which may also facilitate overwintering and virus persistence. Humans most frequently become infected with CTFV when bitten by the adult wood tick (D. andersoni) but probably do not act as a source of re-infection for other ticks. Transmission from person to person has been recorded as the result of blood transfusion. The prolonged viraemia observed in humans and rodents is thought to be due to the intra-erythrocytic location of virions, protecting them from immune clearance.
CTFV is characterized in humans by an abrupt onset of fever, chills, headache, retro-orbital pains, photophobia, myalgia and generalized malaise. Abdominal pain occurs in about 20% of patients. Rashes occur in less than 10% of cases. A diphasic, or even triphasic, febrile pattern has been observed, usually lasting for 5–10 days. Severe forms of the disease, involving infection of the central nervous system, or hemorrhagic fever, or both, have been infrequently observed (nearly always in children under 12 years of age). A small number of such cases are fatal. Congenital infection with CTFV may occur, although the risk of abortion and congenital defects remains uncertain. Antibodies to EYAV have been found in patients with meningoencephalitis and polyneuritis but a causal relationship to the virus has not been established.
CTFV causes leucopoenia in adult hamsters and in about two-thirds of infected humans. Suckling mice, which usually die at 6–8 days post infection, suffer myocardial necrosis, necrobiotic cerebellar changes, widespread focal necrosis and perivascular inflammation in the cerebral cortex, degeneration of skeletal myofibers, hepatic necrosis, acute involution of the thymus and focal necrosis in the retina and in brown fat. The pathologic changes in mice due to CTFV infection (in skeletal muscle, heart and brain), are consistent with the clinical features of human infection, which may include meningitis, meningo-encephalitis, encephalitis, gastro-intestinal bleeding, pneumonia and myocarditis.
CTFV occurs in forest habitats at 4000–10,000 ft elevation in the Rocky Mountain region of North America. Antibodies to the virus have been detected in hares in Ontario and a virus isolate has been reported from Long Island, New York. EYAV appears to be widely distributed in Europe. An Eyach-like virus has also been identified from ticks in the USA. A novel coltivirus designated Taï Forest reovirus (TFRV) has been identified from the blood of a free-tailed bat in Côte d’Ivoire and represents a novel species within the genus.
Antigenicity
There is little cross-reaction in virus neutralization tests between isolates of CTFV (from North America) and EYAV (from Europe). CTFV-Ca from a hare, collected in California in 1976, shows some one-way cross-reaction in serum neutralization tests with EYAV, but is clearly distinguishable. Distinct serotypes of both CTFV and EYAV have been reported.
Species demarcation criteria
In addition to the other general criteria used throughout the family, members of a species in the genus Coltivirus may be identified by:
- RNA cross-hybridization assays: within a single species, RNA sequences that have more than 74% similarity will hybridize at 36°C below the Tm of the fully base-paired duplex.
- Sequence analysis: Nucleotide identity of >89% in Seg12; amino acid identities of >55%, >57% and >60% respectively in the more variable VP6, VP7 and VP12 proteins.
Related, unclassified viruses
| Virus name | Accession number | Abbreviation |
| Salmon River virus* | SRV |
Virus names and virus abbreviations, are not official ICTV designations.
* Reported as a serotype distinct from CTFV (Attoui et al., 2005).

