Family: Phenuiviridae
Genus: Phlebovirus
Distinguishing features
Phleboviruses infect mammals including livestock and humans, and are transmitted by infected mosquitoes, phlebotomine sandflies or ticks. Several members of the genus Phlebovirus cause large epizootic epidemics in livestock and humans. Eighty-two species for 98 viruses are assigned to the genus Phlebovirus. The phlebovirus genome encompasses three single-stranded RNA segments, encoding four structural proteins: a large protein (L), two external glycoproteins (Gn and Gc) and a nucleocapsid protein (N), as well as two non-structural proteins (NSm and NSs). Based on well-supported Maximum Likelihood or Maximum Clade Credibility trees inferred from complete L protein sequences, viruses classified in the genus Phlebovirus form a monophyletic cluster clearly distinguished from other phenuivirids (Palacios et al., 2011a, Palacios et al., 2011b, Ikegami 2012, Elliott and Brennan 2014, Palacios et al., 2014, Alkan et al., 2015, Palacios et al., 2015, Nunes-Neto et al., 2017).
Virion
Morphology
Virions have a uniform size of 80–120 nm in diameter and have surface glycoprotein projections of 9 nm which are embedded in a lipid bilayer envelope 5–10 nm thick. Mature virions contain three major structural proteins of approximately 65, 55 and 27 kDa that correspond to the Gn, Gc and N proteins of phenuivirids (Ikegami 2012).
Nucleic acid
The phlebovirus genome encompasses three single-stranded segments composed of negative-sense or ambisense RNAs. The terminal nucleotides of each segment occur in a canonical, conserved sequence (in coding sense) 5′-ACACAAAGAC…GUCUUUGUGU-3′ and may form the panhandle structures typical of other members of the class Bunyaviricetes (Table 2 Phenuiviridae). The L segment (6.3–6.5 kb) encodes a protein with a predicted molecular mass of 228–242 kDa that shares the bunyaviral RNA-directed RNA polymerase (RdRP) domain. The M segment (3.3–4.8 kb) encodes a protein of 114–163 kDa that is homologous with the phlebovirus nonstructural protein NSm, glycoprotein G1 and G2 sequences, and the phlebovirus glycoprotein C-terminal Ig-like domain. The S segment (1.4–3.3 kb) encodes two proteins of 16–37 kDa and 16–39 kDa that are homologous with the tenuivirus/phlebovirus N domain and Rift Valley fever virus non-structural protein-like domain, respectively (Table 3 Phenuiviridae).
Genome organization and replication
The phlebovirus genome encompasses three single-stranded RNA segments (Figure 1 Phlebovirus). The L, M, and S segments encode L, a glycoprotein precursor (comprising Gn and Gc) and N, respectively. The M segment has a preglycoprotein coding region that codes for NSm (14–56 kDa), followed by the Gn (47–79 kDa) and Gc (45–65 kDa) glycoproteins. Some phleboviruses do not encode NSm in the glycoprotein precursor. The S segment exhibits an ambisense coding strategy, with two ORFs, N and NSs, separated by a noncoding intergenic region that potentially forms a long A/U rich stem-loop structure. The NSm proteins of phleboviruses can act as apoptosis antagonists. The NSs proteins of phleboviruses act as interferon antagonists (Palacios et al., 2014, Palacios et al., 2015, Nunes-Neto et al., 2017, Marklewitz et al., 2020). Replication, morphogenesis, assembly, and budding are described in ‘Genome organisation and replication’ section of the family page.
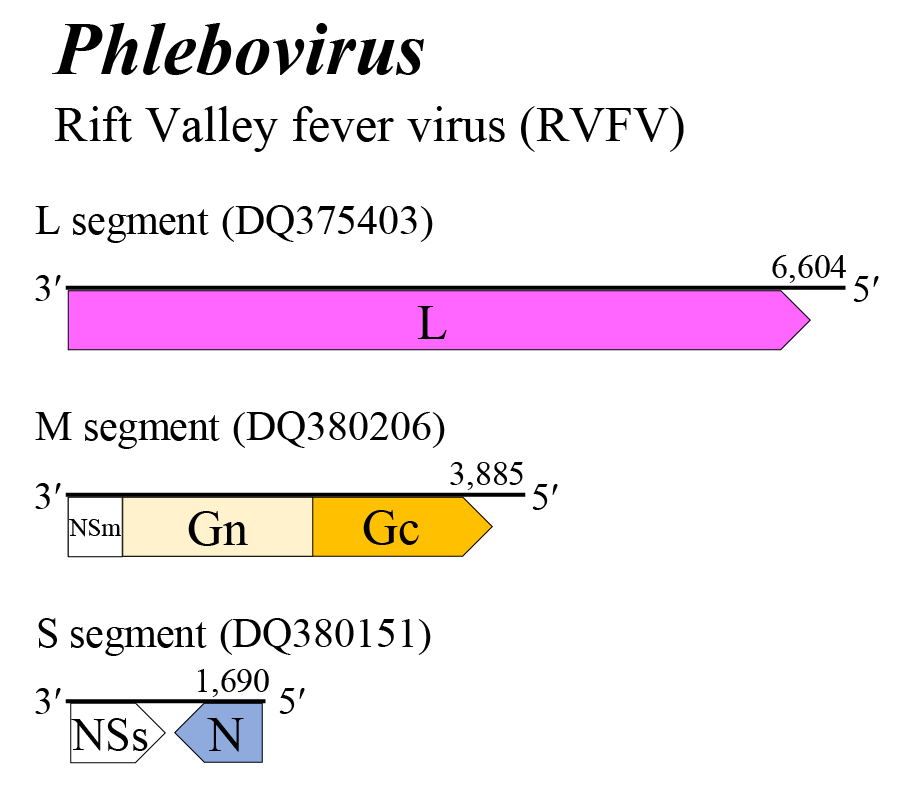 |
| Figure 1 Phlebovirus. Genome organization of a phlebovirus. Coloured boxes depict ORFs that encode N, nucleocapsid protein; Gn and Gc, external glycoproteins; and L, large protein. White boxes depict ORFs that encode NSm, non-structural protein on the M segment and NSs, non-structural protein on the S segement. |
Biology
Host range
Several members of the genus Phlebovirus cause large epizootic epidemics in livestock and humans. Outbreaks of Rift Valley fever were initially recognized as “abortion storms” in herds of pregnant ruminants in the Great Rift Valley, Kenya, in 1930 (Daubney and Hudson 1931). Toscana virus, first isolated in 1971 from phlebotomine sand flies in the Tuscany region of Italy, causes febrile illness and more severe neuroinvasive diseases in humans (Verani et al., 1982). Phleboviruses infect mammals and are transmitted by infected mosquitoes, phlebotomine sandflies or ticks. Hosts of phleboviruses include cattle, camels, goats, sheep, dogs, rodents [Acomys cahirinus (E. Geoffrey, 1803), Aethomys hindei (Thomas, 1902), Mus striatus (Linnaeus, 1758), Neotoma micropus (Baird, 1885), and Proechimys guyannensis (Geoffroy, 1803)], two-toed sloths [Choloepus didactylus (Linnaeus, 1758) and C. hoffmanni (Peters, 1858)], opossums (Virginia opossum (Kerr, 1792)] and humans. Several phleboviruses cause abortion and infection often manifests as congenital malformations in livestock and/or is associated with fatal febrile illness and occasionally encephalitides in humans. In the case of RVFV infection, 40–100% of pregnant ewes abort, and the fetuses often have malformations. Newborn lambs suffer peracute disease, characterized by necrotic hepatitis with 95–100% mortality. Pregnant cattle infected with RVFV frequently abort, and overall mortality can reach 30%. In most human cases, the disease is characterized by a febrile illness, which progresses to more serious complications in 1–2% of infected individuals, with symptoms including hepatitis, encephalitis, retinitis, blindness, or a hemorrhagic syndrome and a resulting hospitalized case fatality rate of 10 to 20% (Palacios et al., 2011a, Palacios et al., 2011b, Ikegami 2012, Elliott and Brennan 2014, Palacios et al., 2014, Alkan et al., 2015, Palacios et al., 2015, Nunes-Neto et al., 2017).
Transmission
Phleboviruses are transmitted by particular arthropod vectors. The major vectors are flies of the species Culex antennatus (Becker, 1903), Nyssomyia trapidoi (Fairchild & Hertig, 1952), Phlebotomus major (Annandale, 1910), P. papatasi (Scopoli, 1786), P. perniciosus (Newstead, 1911) and Psychodopygus panamensis (Shannon, 1926), and ticks of the species Ixodes persulcatus (Schulze, 1930). Phleboviruses can be transmitted transovarially by virus-infected female arthropods to their offspring, and through sperm from virus-infected males. In addition to arthropod vector transmission, mammals can become infected through contact with the blood or body fluids of infected animals. Person-to-person transmission has also been reported for people caring for infected patients (Charrel et al., 2009, Ikegami 2012, Alkan et al., 2013, Ly et al., 2017, Matsuno et al., 2018, Marklewitz et al., 2019).
Species demarcation criteria
The criteria demarcating species in the genus are:
• Less than 95% identity in the amino acid sequence of the RdRP
Relationships within the genus
Phylogenetic relationships across the genus have been established from maximum likelihood trees generated from full sequences of L proteins (Figure 2.Phlebovirus).
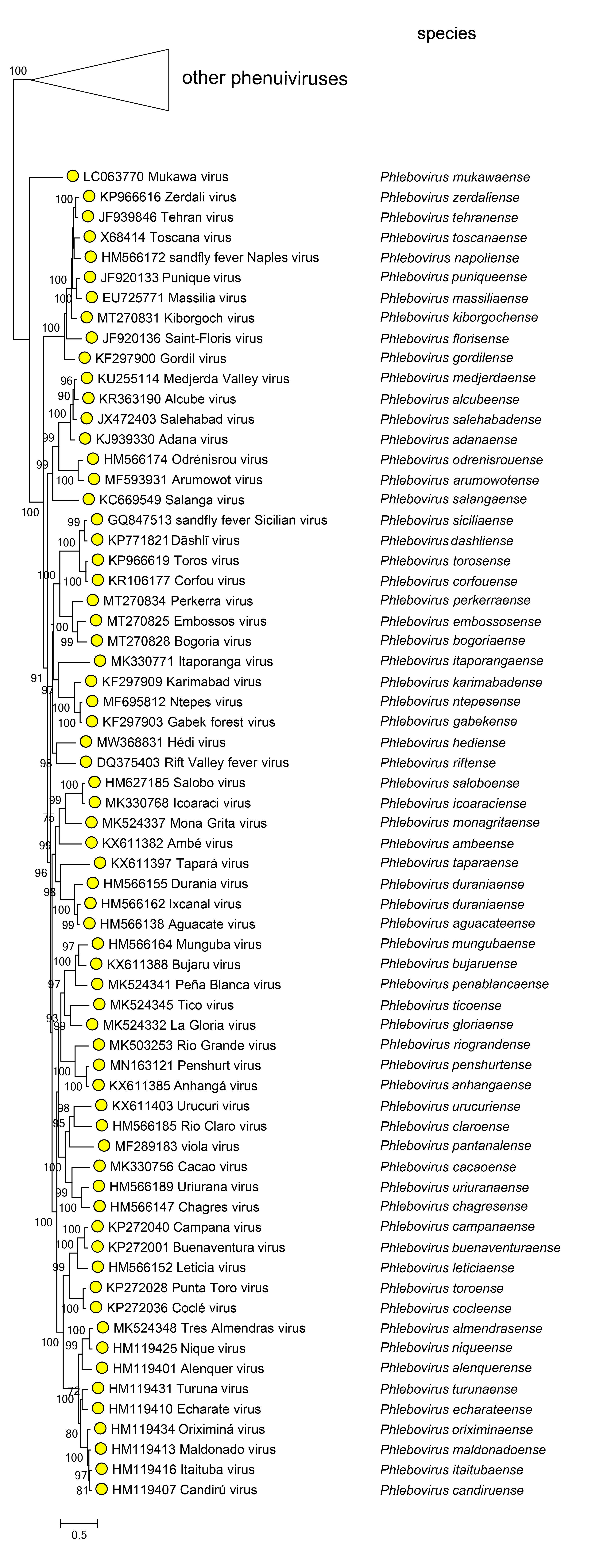 |
| Figure 2 Phlebovirus. Phylogenetic analysis of phleboviruses. Phylogenetic reconstruction is based on a MAFFT-alignment of the RdRP amino acid sequences of phenuivirids using E-INS algorithm. The ML phylogenetic tree was inferred using RaxML-NG performing 1,000 bootstrap replicates. Trees were inferred under the WAG substitution model. Tree branches are proportional to genetic distances between sequences and the scale bars at the bottom indicate substitutions per amino acid. Branches for viruses in other genera are collapsed; an expanded version of this tree is available on the family page (Figure 3 Phenuiviridae). |
Related, unclassified viruses
| Virus name | Accession number | Virus abbreviation |
| Alxa tick phlebovirus | L: OP313008; M: OP313009; S: OP313010 | ATPV |
| Arboledas virus# | ADSV | |
| Arrábida virus | L: KP863799; M: KP863800; S: KP863801 | ARRV |
| Balambala tick virus | L: MW561967; S: MW561971 | BaTiV |
| Chios virus | L: AY293623* | CHOV |
| Gissar virus | L: KJ425423*; M: KJ425424*; S: KJ425425 | GISV |
| Khasan virus | L: KF892046; M: KF892047; S: KF892048 | KHV |
| Komandory virus | L: KF892049; M: KF892050; S: KF892051 | KOV |
| Lanjan virus | L: KM370974* | LanV |
| Mariquita virus$ | MRQV | |
| Okutama tick virus | L: LC753199; S: LC753204 | OKTV |
| Ponticelli virus I | L: KX388211; M: KX388212; S: KX388213 | PONVI |
| Provencia virus | L: GU446659* | PROV |
| Qīngdǎo tick phlebovirus | L: OQ513651; M: OQ513652; S: OQ513653 | QTPV |
| sandfly Nepal virus | L: MK598070*; M: MK598071*; S: MK598072* | SNV |
| Sara tick phlebovirus | L: ON408148; S: ON408149 | STPV |
| Utique virus | L: OM362902* | UtiV |
* Sequences do not comprise the complete genome segment.
Virus names and virus abbreviations are not official ICTV designations.

