Subfamily: Parvovirinae
Genus: Amdoparvovirus
Distinguishing features
These viruses infect small carnivores including mink and other mustelids, foxes, skunk, and raccoons. Several members establish persistent infections that can lead to immune-complex disease. Four species are recognized, Carnivore amdoparvovirus 1, Carnivore amdoparvovirus 2, Carnivore amdoparvovirus 3 and Carnivore amdoparvovirus 4, each of which includes a single virus (respectively, Aleutian mink disease virus (ADV), gray fox amdovirus (GFAV), raccoon dog and fox amdoparvovirus (RFAV) and skunk amdoparvovirus (SKAV)). The VP1 N termini of amdoparvoviruses are unusually short and there is no evidence of a phospholipase 2A enzymatic core. Phylogenetically they appear most closely related to members of genus Protoparvovirus (Figure 6B. Parvoviridae).
Virion
See discussion under family description.
A cryo-EM structure of recombinant ADV-G VP2-only particles has been published (McKenna et al., 1999).
Genome organization and replication
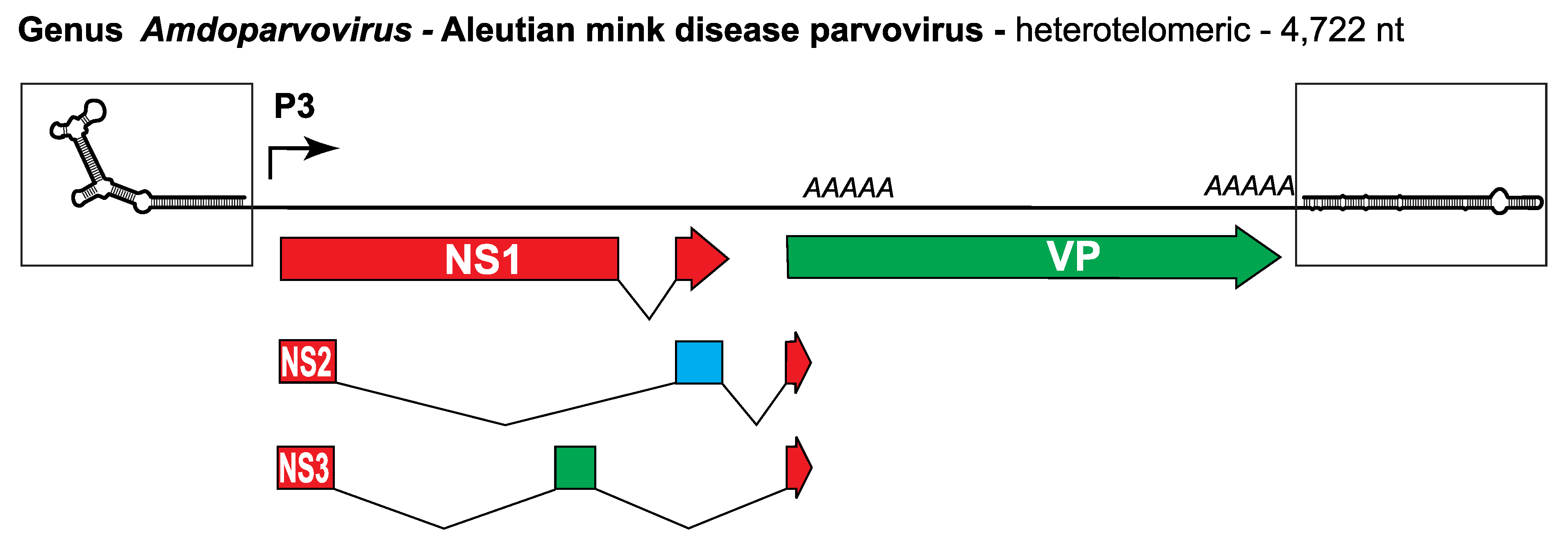
ADV has a heterotelomeric genome of around 4.7 kb (Figure 1. Amdoparvovirus) and packages negative-sense DNA predominantly (~95%). A transcriptional promoter near the left end of the genome generates a single pre-mRNA, which is variably processed using 3 different splicing patterns and 2 alternative polyadenylation sites (Qiu et al., 2006a). This yields six distinct mRNA classes, two that express NS1, two expressing NS2 or the capsid proteins VP1 or VP2, and the remaining two expressing NS3 or VP2 (Huang et al., 2014b). Ancillary proteins NS2 and NS3 are both required for productive infection. Caspase processing of NS1 is required to release a form of the protein that can be transported into the nucleus, which is essential for viral replication (Best et al., 2003).
|
|
|
Figure 1. Amdoparvovirus. Genetic strategy of Aleutian mink disease parvovirus (ADV), genus Amdoparvovirus. The heterotelomeric genome of ADV is shown as a single line terminating in boxed hairpin structures that are magnified relative to the rest of the genome to show potential secondary structure: the hairpin on the left is 116 nt, that on the right is 242 nt. Major open reading frames (ORFs) encoding proteins are shown as arrowed boxes, some of which are linked by splicing to create ancillary proteins. Regions of ORF1 shaded red encode the entire replication initiator protein NS1 and contribute the first and third introns of both NS2 and NS3. Sections of ORF3 shaded green encode the capsid proteins, VP, while sections unique to ancillary protein NS2 are blue. Regions of ORF2 unique to NS3 are yellow. Solid arrows represent transcriptional promoters; AAAAA indicates polyadenylation sites. |
Biology
ADV is of economic importance in mink farming, particularly affecting inbred mink with the desirable Aleutian (grey) coat colour. It is the only member of this genus that has been purified and studied in culture at the molecular level. ADV virulence differs markedly between virus strains and with the genetics of the host animal. The most successful laboratory-adapted virus, ADV-G (JN040434), is apathogenic even in Aleutian mink. Nevertheless, ADV-G has many characteristics that limit its infectivity in vitro and might be expected to support a persistent lifestyle in vivo. For example, its capsid activates host cell caspases that degrade most of the newly synthesized capsid protein at specific cleavage sites, but if these sites are inactivated by mutagenesis, virus production is enhanced (Cheng et al., 2010). Since parvoviruses have high mutation rates, caspase sites could be modified rapidly in vivo if that promoted viral success, suggesting that ADV is specifically adapted to support an inefficient but relatively unobtrusive life style.
Virulent ADV virus strains, such as Utah 1 (KU513988), evoke high mortality rates in Aleutian mink, where they cause a progressive immune complex-associated syndrome called plasmacytosis (or Aleutian disease) that leads to glomerulonephritis and is driven by extremely high levels of antiviral antibody. In newborn animals, infection is permissive but causes interstitial pneumonitis that is often fatal. In contrast, when these high virulence strains infect non-Aleutian farmed or wild mink, or other mustelids such as weasels, ferrets, badgers, or otters, they are sometimes pathologenic but are more often asymptomatic and non-persistent (Canuti et al., 2015). SKAV, the predominant skunk virus, also sometimes infect mink. However, it is highly prevalent in striped skunk in Canada and parts of North America, where it also gives rise to immune-complex-like lesions in some animals, although infection is generally subclinical (Canuti et al., 2017). Multiple strains of ADV and SKAV have been documented, even within the same geographic area, and infection of the same animal with disparate strains is common. This rampant diversification appears to be driven by intensive mink farming practices that facilitate the occurrence of co-infections, favouring viral recombination, but spreads through surrounding wild populations (Canuti et al., 2016). Available epidemiology is limited for GFAV (Li et al., 2011) and RFAV (Shao et al., 2014), but these are also potential pathogens, producing disease spectra compatible with immune-complex disorders.
Antigenicity
Anti-capsid antibodies directed against ADV can neutralize infection in tissue culture, but in animals they form infectious immune complexes with the virus that exacerbate disease by binding to Fc receptors on the surface of circulating host macrophages, leading to enhanced virus uptake and infectivity (Bloom et al., 2001).
Species demarcation criteria
Viruses within a species are monophyletic and encode replication initiator proteins (called NS1 or Rep1, 68, or 78) that show >85% amino acid sequence identity.
Related, unclassified viruses
|
Virus name |
Accession number |
Virus abbreviation |
|
red panda amdoparvovirus |
RpAPV |