Family: Picornaviridae
Alexander E. Gorbalenya, Tapani Hovi, Nick J. Knowles, Michael Lindberg, Steven Oberste, Ann C. Palmenberg, Gábor Reuter, Peter Simmonds, Tim Skern, Caroline Tapparel, Katja Wolthers, Patrick Woo and Roland Zell
Corresponding author: Roland Zell ([email protected])
Edited by: Nick J. Knowles and Peter Simmonds
Posted: October 2017, updated November 2019 & September 2020
PDF: ICTV_Picornaviridae.pdf
Summary
The Picornaviridae is a family of small, icosahedral viruses with single-stranded, highly diverse positive-sense RNA genomes (Table 1. Picornaviridae). Characteristic features of all members of the family are three capsid proteins with β-barrel folding, polyprotein processing by virus-encoded cysteine proteinase(s), and replication by an RNA-directed RNA polymerase with a YGDD sequence motif. The family comprises 63 genera containing 147 species, but many viruses are presently awaiting classification. Picornaviruses may cause subclinical infections of humans and animals or conditions ranging from mild febrile illness to severe diseases of heart, liver and the central nervous system.
Table 1. Picornaviridae. Characteristics of members of the family Picornaviridae.
| Characteristic | Description |
| Typical member | poliovirus 1 Mahoney (V01149), species Enterovirus coxsackiepol |
| Virion | Non-enveloped, 30–32 nm virions comprising 60 protomers |
| Genome | 6.7–10.1 kb of positive-sense, non-segmented RNA with a poly(A) tail |
| Replication | RNA synthesis occurs in reorganized cytoplasmic replication organelles containing non-structural proteins derived from the 2BC-P3 region of the encoded polyprotein; RNA structures at the 5′- and 3′-ends of the genome direct initiation of RNA synthesis and uridylated 3B serves as primer for synthesis of both RNA strands |
| Translation | Directly from genomic RNA containing an internal ribosomal entry site (IRES) |
| Host range | Vertebrates (at least six of the seven classes) |
| Taxonomy | Realm Riboviria, kingdom Orthornavirae, phylum Pisuviricota, class Pisoniviricetes, order Picornavirales; 5 subfamilies, 68 genera containing 159 species |
Virion
Morphology
Virions consist of a capsid, with no envelope, surrounding a core of ssRNA. Available crystal structures indicate that particles are 30–32 nm in diameter. Electron micrographs reveal no projections on virions of most picornaviruses, the virus particle appearing as an almost featureless sphere; however, kobuviruses show a surface structure which is distinct from small round structured viruses (astroviruses and caliciviruses) (Figure 1. Picornaviridae). The capsid is composed of 60 identical units (protomers). Those picornaviruses with four capsid proteins have three surface proteins, 1B, 1C and 1D, of 24–41 kDa, and an internal protein, 1A of 5.5–13.5 kDa; however, many picornaviruses have three capsid proteins as 1AB (VP0) remains uncleaved. Total protomer is 80–97 kDa. Proteins 1A, 1B, 1C and 1D are also commonly named VP4, VP2, VP3, and VP1, respectively. Proteins 1B, 1C, 1D and the uncleaved 1AB each possess a core structure comprising an eight-stranded β-sandwich ("β-barrel"). Capsid protein (CP) sequences in many genera reveal similarities to those in the "rhv-like" superfamily of proteins, and may contain a conserved "inhibitor binding site" which, in the case of some rhino- and enteroviruses, has been demonstrated to bind active antivirals. The β-barrels pack together in the capsid with T=1, pseudo T=3, icosahedral symmetry; these structural features are shared by all members of the order Picornavirales with solved atomic structures, e.g. cricket paralysis virus (Dicistroviridae), infectious flacherie virus (Iflaviridae) and the comoviruses cowpea mosaic virus, bean pod mottle virus and red clover mottle virus.
Members of different genera differ in the external loops that interconnect the β-strands. These loops account for differences in surface relief of members of each genus (Figure 1. Picornaviridae) and in the thickness of the capsid wall. Assembly occurs via pentameric intermediates (pentamer = five protomers). Proteins within each pentamer are held together by an internal network formed from the N-termini of the three major CPs, the C-termini lying on the outer capsid surface. In members of genera where the 1AB is cleaved in the complete virion, empty capsids, which are produced by some picornaviruses, are very similar to virions, except that 1A and 1B are normally replaced by the uncleaved precursor, 1AB.
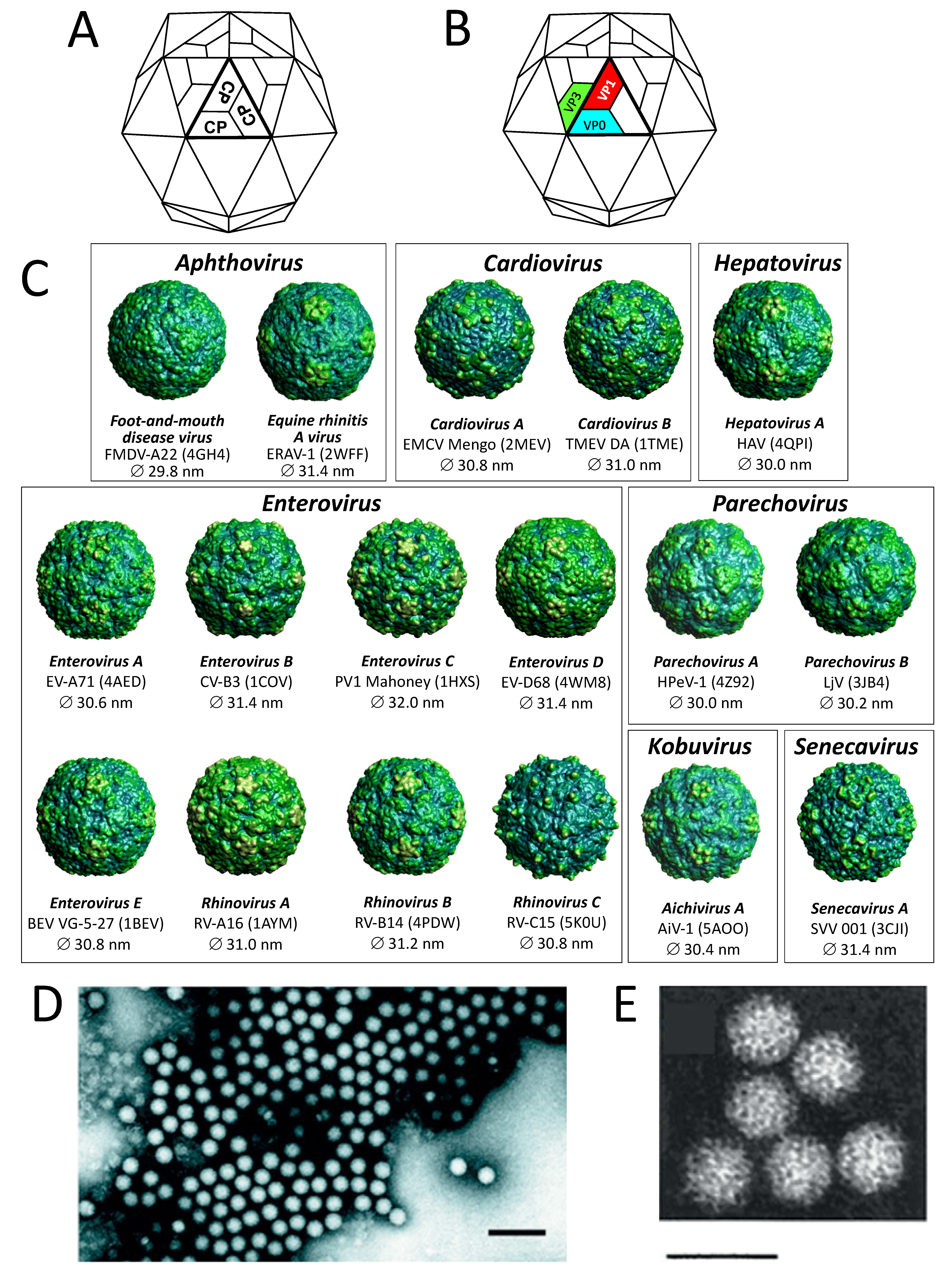 |
| Figure 1.Picornaviridae. (A) Schematic of the icosahedral T=3 capsid (small stellated dodecahedron). Each of the 60 facets comprises three protomers (protomer = one copy of the capsid protein CP) and matches with the crystallographic asymmetric unit (thick lines). (B) Schematic of the icosahedral T=1 pseudoT=3 capsid. The picornavirus protomer (comprising one copy each of the capsid proteins VP1, VP0, VP3) and the facets of the T=3 capsid are distinct. In fact, three protomers constitute one of 20 facets of the icosahedron (thus T=1). Where VP0 is cleaved, the fourth capsid protein (VP4) is located about the internal surface of the pentameric apex of the icosahedron. (C) Pictures of 17 picornavirus structures (from VIPERdb http://viperdb.scripps.edu (Carrillo-Tripp et al., 2009)). Indicated are genus, species, virus name abbreviation, PDB entry and the virion's average diameter. (D) Negative-contrast electron micrograph of poliovirus (PV) particles; the bar represents 100 nm (courtesy of Ann C. Palmenberg). (E) Negative-contrast electron micrograph of Aichi virus A1 (genus Kobuvirus) showing surface structure; the bar represents 50 nm (courtesy of Teruo Yamashita). |
Physicochemical and physical properties
The molecular weight of picornavirus virions ranges from 8 × 106 – 9 × 106 with a sedimentation rate (S20w) of 140–165S (for empty particles S20w is 70–80S). Their buoyant density in CsCl is 1.33–1.45 g cm-3, depending on the genus. Some species are unstable below pH 7; many are less stable at low ionic strength than at high ionic strength. Virions are insensitive to ether, chloroform, or non-ionic detergents. Viruses are inactivated by light when grown with, or in the presence of photodynamic dyes such as neutral red or proflavin. Thermal stability varies with viruses as does stabilization by divalent cations.
Nucleic acid
Virions contain one molecule of positive-sense ssRNA of 6.7–10.1 kb and possessing a single long ORF; cadiciviruses (genus Dicipivirus), however, have two ORFs, with translation of each directed by a separate internal ribosomal entry site (IRES). A poly(A) tail, heterogeneous in length, is located after the 3′-terminal heteropolymeric sequence. A small protein, VPg (c. 2.2 to 3.9 kDa), is linked covalently to the 5′-terminus. The untranslated regions (UTRs) at both termini contain regions of secondary structure which are essential to genome function. The long 5′-UTR (0.5–1.5 kb) includes a 5′-terminal domain involved in replication (e.g. the poliovirus "clover-leaf") and an IRES of 220–450 nt upstream of the translational start site; most picornaviral IRES elements can be assigned to one of several types (I to V, IGR-IRES), according to their secondary structure. Between the 5′-terminal domain and the IRES there may be one, or more, pseudoknots. A poly(C) tract is found in the 5′-UTR of foot-and-mouth disease viruses, encephalomyocarditis viruses and possibly porcine teschoviruses. The 3′-UTR, which may also contain a pseudoknot, ranges from 25 to nearly 800 nt.
Proteins
In addition to the major CPs, 1A, 1B, 1C and 1D, and 3B (VPg), described above, small amounts of 1AB (VP0) are commonly seen in lieu of one or more copies of 1A and 1B. Protein 1A is small in craheliviruses, hepatoviruses, fipiviruses, gruheliviruses, roheliviruses and tremoviruses, and 1AB is uncleaved in members of the Aalivirus, Ampivirus, Aquamavirus, Avihepatovirus, Avisivirus, Crohivirus, Gallivirus, Hemipivirus, Kobuvirus, Kunsagivirus, Limnipivirus, Livupivirus, Ludopivirus, Megrivirus, Mischivirus, Myrropivirus, Orivirus, Oscivirus, Parechovirus, Pasivirus, Passerivirus, Pemapivirus, Poecivirus, Potamipivirus, Rosavirus, Sakobuvirus, Salivirus, Shanbavirus, Sicinivirus, Symapivirus and Tropivirus genera, as well as in a number of unclassified picornaviruses.
Lipids
Some picornaviruses carry a sphingosine-like molecule ("pocket factor") in a cavity ("pocket") located inside 1D. Protein 1A, where present, generally has a molecule of myristic acid covalently attached to the amino terminal glycine. No myristoylation signal is found in the N-terminal VP4 and VP0 sequence of avihepatoviruses, dicipiviruses, kobuviruses, parechoviruses and tremoviruses.
Carbohydrates
None of the viral proteins are glycosylated.
Genome organization and replication
The virion RNA is infectious (Colter et al., 1957, Alexander et al., 1958) and serves as both the genome and the viral mRNA. Initiation of protein synthesis is stimulated by the IRES. Translation of the single ORF produces the polyprotein precursor (216–277 kDa) to the structural proteins (derived from the P1 region of the genome) and the non-structural proteins (from the P2 and P3 regions) (Figure 2. Picornaviridae). In many viruses P1 is preceded by a leader protein (L). In dicipiviruses ORF1 encodes the precursor of the structural proteins (corresponding to the P1 region), whereas ORF2 encodes the functional protein precursor (P2 and P3 regions).
The polyprotein is cleaved to functional proteins by virus-encoded proteinases. Intermediates are denoted by letter combinations (e.g. 3CD, the uncleaved precursor of 3C and 3D). The viral proteinases are as follows (Figure 2. Picornaviridae): 3Cpro, a chymotrypsin-like cysteine protease encoded by all picornaviruses, performs most of the cleavages; 2Apro related to 3Cpro is responsible for few cleavages in enteroviruses, and possibly boosepiviruses, diresapiviruses, felipiviruses, paraboviruses, raboviruses and sapeloviruses; Lpro residing in a leader protein is a papain-related cysteine protease that releases itself from polyprotein in aphthoviruses, erboviruses and possibly mosaviruses; L protein of duck picornavirus (anativirus A1) is suspected to be a trypsin-like protease. Also, the 2A of aaliviruses, ailuriviruses, aphthoviruses, aquamaviruses, avihepatoviruses, avisiviruses, bopiviruses, cardioviruses, cosaviruses, crohiviruses, erboviruses, grusopiviruses, hunniviruses, kunsagiviruses, limnipiviruses, malagasiviruses, mischiviruses, mosaviruses, parechoviruses except members of the species Parechovirus A, pasiviruses, potamipiviruses, senecaviruses, shanbaviruses, teschoviruses, torchiviruses and of a number of unclassified picornaviruses contains an NPG↓P motif that mediates cotranslational termination-reinitiation of RNA translation (↓ = co-translational stop-go site) and acts only in cis (2Anpgp). Conserved sequence motifs are found in the 2C helicase (Walker A motif: GxxGxGKS), 3C proteinase (GxCGx10-15GxH) and 3D polymerase (KDE, DxxxxD, PSG, YGDD, FLKR).
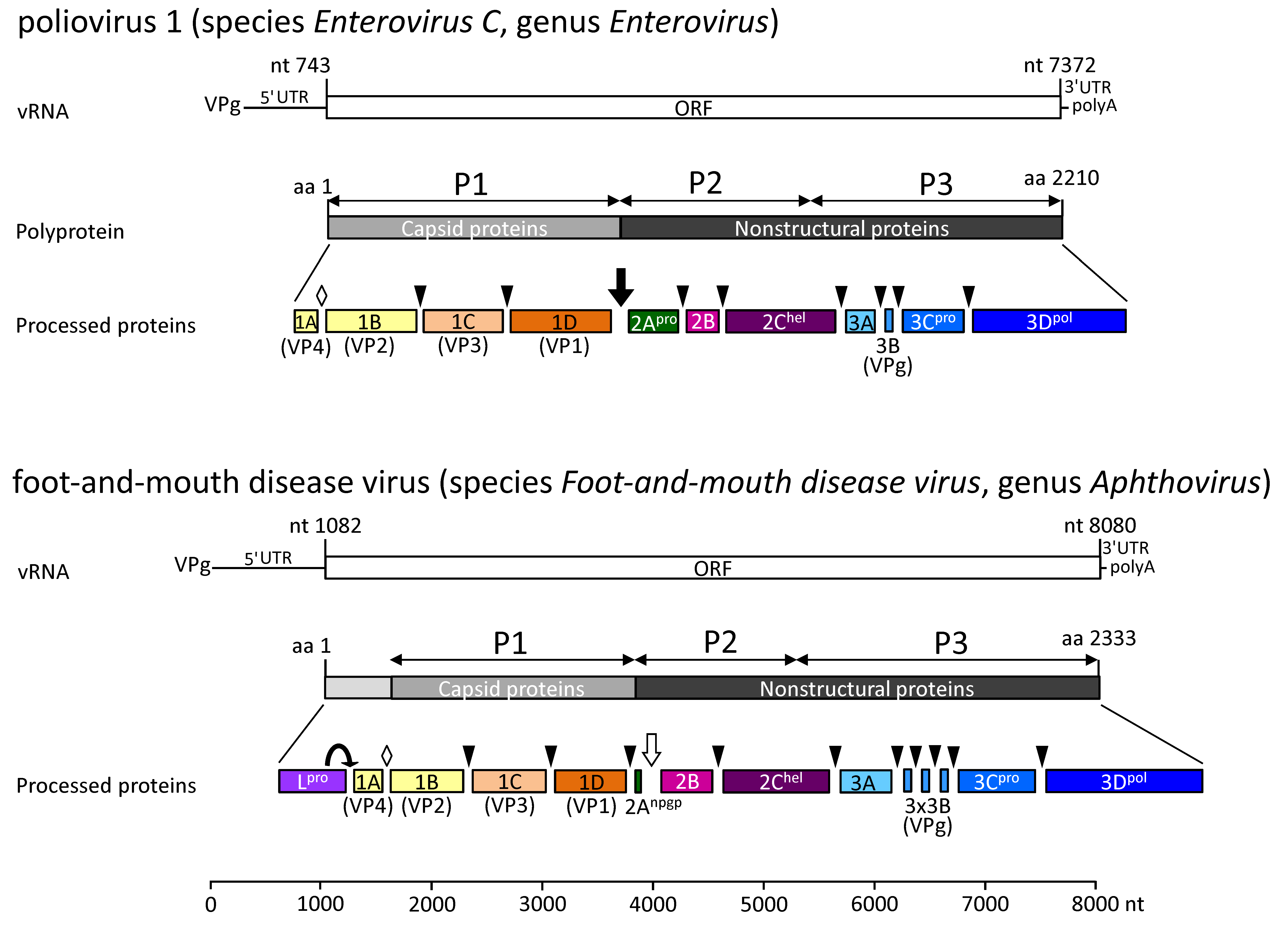 |
| Figure 2. .Picornaviridae. Polyprotein processing of enteroviruses and aphthoviruses. Viral RNA (vRNA) is polyadenylated and covalently linked to a virus-encoded protein (VPg) at its 5′-end. A single open reading frame (ORF) encoding a polyprotein is flanked on either side by untranslated regions (UTRs). Release of the capsid protein precursor (P1) is facilitated by 2Apro of the enteroviruses (black arrow) or by an NPG↓P motif at the C-terminus of 2A of foot-and-mouth disease virus (white arrow). P2 and P3 polypeptides are precursors of the nonstructural proteins necessary for genome replication. Further polyprotein processing is conducted by 3Cpro (cleavage sites indicated by arrow heads). The leader proteinase Lpro releases itself from the P1 by cleavage at its own C-terminus. Processing of VP0, the precursor of VP4 and VP2, is thought to be autocatalytic and occurs within the provirion at virion maturation (white diamond). Numbering refers to the genomes of strains poliovirus 1 (V01149) and foot-and-mouth disease virus (FMDV) O6 (AY593829). As the actual length of the poly(C) tract in the 5′-UTR of FMDV O6 has not been determined, the position of the start and stop codons may vary by 100–200 nt. |
Polyprotein processing has only been studied for enteroviruses and aphthoviruses. Viral RNA (vRNA) is polyadenylated and covalently linked to a virus-encoded protein (VPg) at its 5′-end. A single open reading frame (ORF) encoding a polyprotein is flanked on either side by untranlated regions (UTRs). Release of the capsid protein precursor (P1) is facilitated by 2Apro of the enteroviruses (black arrow) or by an NPG↓P motif at the C-terminus of 2A of foot-and-mouth disease virus (white arrow). P2 and P3 polypeptides are precursors of the nonstructural proteins necessary for genome replication. Further polyprotein processing is conducted by 3Cpro (cleavage sites indicated by arrow heads). The leader proteinase Lpro releases itself from the P1 by cleavage at its own C-terminus. Processing of VP0, the precursor of VP4 and VP2, is thought to be autocatalytic and occurs within the provirion at virion maturation (white diamond). Numbering refers to the genomes of strains poliovirus 1 (V01149) and foot-and-mouth disease virus (FMDV) O6 (AY593829). As the actual length of the poly(C) tract in the 5′-UTR of FMDV O6 has not been determined, the position of the start and stop codons may vary by 100–200 nt.
Picornaviruses exhibit a modular genome organization (Figure 3. Picornaviridae). Certain genetic elements of the 5′- and 3′-UTR and gene regions are thought to have been exchanged between members of different genera. There may be one or more gene regions encoding various 2A proteins. Beside 2Apro and the aphthovirus-like 2Anpgp, 2A may have the H-box/NC sequence motif of a family of cellular proteins involved in the control of cell proliferaton [aaliviruses, avihepatoviruses, avisiviruses, crohiviruses, fipiviruses, galliviruses, members of the species Grusopivirus A, hemipiviruses, kobuviruses, livupiviruses, megriviruses, myrropiviruses, parechoviruses, passeriviruses, pemapiviruses, potamipiviruses, rosaviruses, sakobuviruses, saliviruses, shanbaviruses, siciniviruses, symapiviruses, tremoviruses, tropiviruses and several unclassified picornaviruses; (Hughes and Stanway 2000)] or similarity to the AIG1-type guanine binding domain, a P-loop NTPase with GxxGxGKS motif (aaliviruses, avihepatoviruses, avisiviruses); their function in virus replication is unclear. The 2B and 3A gene regions vary greatly among the genera, although functionally they may be homologous. Some intermediates are stable and serve functions distinct from those of their cleavage products (e.g. cleavage of poliovirus P1 by 3CDpro, not by 3Cpro). Where it occurs, the cleavage of 1AB, which accompanies RNA encapsidation, is thought to be autocatalytic, but the precise mechanism is unknown. For enteroviruses, an additional polypeptide of 67 amino acids, UP, encoded by an upstream open reading frame (uORF) has been proposed (Lulla et al., 2019).
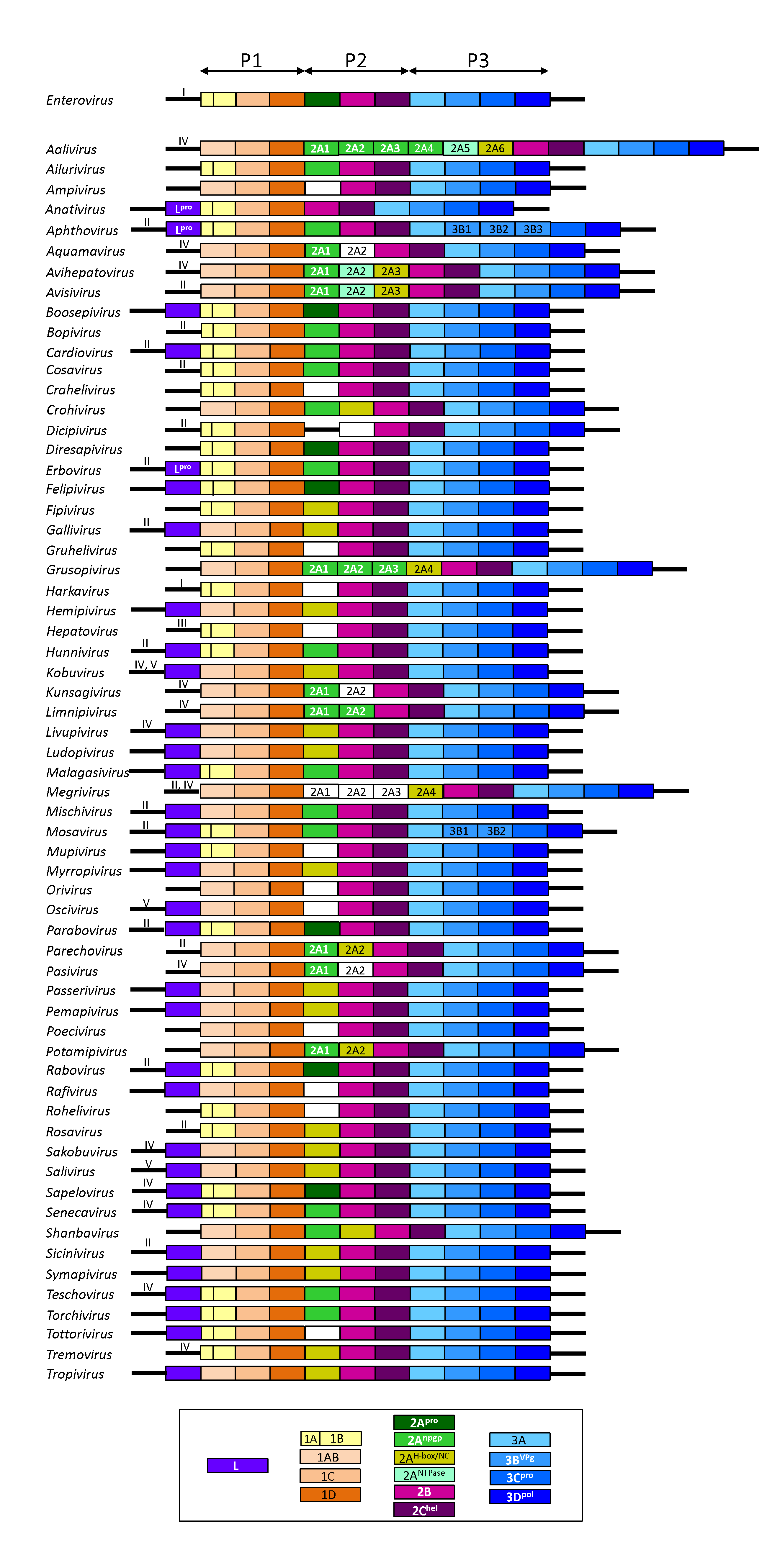 |
| Figure 3. .Picornaviridae. Schematic genome organization of representative members of 63 picornavirus genera. The paradigmatic genus Enterovirus is shown in the first row; the remaining picornavirus genera are presented in alphabetical order. Open reading frames are represented by coloured boxes. Colours indicate function of the processed proteins; functions are explained in the box below. 2A genes with unknown function are indicated by white boxes. The function of the L protein is unknown except for the aphthoviruses and erboviruses where it is a papain-like protease. Open reading frames are flanked by 5′- and 3′-untranslated regions. Where known, IRES type (I to V) is given by Roman numerals. For clarity, gene regions of the open reading frames and untranslated regions are not drawn to scale. Foot-and-mouth disease virus of genus Aphthovirus has three copies of 3B (VPg) whereas the aphthoviruses bovine rhinitis A virus, bovine rhinitis B virus 1 and equine rhinitis A virus (not shown) have only one. 2A of Boone cardioviruses (Cardiovirus C, not shown) is homologous to the respective proteins of other cardioviruses but has no NPG↓P motif. Members of Megrivirus have one to four 2A proteins up to three of which have unknown function. Two types of IRESs (IV and V) are found in kobuviruses. Members of Enterovirus K have a second 2Apro with an active site serine residue. Various members of Enterovirus G have a porcine torovirus-like cysteine protease-encoding region inserted between the 2C and 3A gene regions. The enterovirus uORF which was recently proposed is not shown. Members of Grusopivirus B lack the 2Anpgp and 2AH-box/NC proteins but have a 2A with unknown function. Kunsagiviruses may have one to three copies of 2Anpgp. All members of Parechovirus A lack the 2Anpgp protein. |
A typical picornavirus genome layout may be represented by the following:
VPg+5′-UTR-[(L-)1A-1B-1C-1D/2A-2B-2C/3A-3B-3C-3D]-3′-UTR-poly(A)
Where “[” and “]” define the extent of the polyprotein-coding region, “/” represents primary cleavages and “-” represents the final cleavages. Where a particular polypeptide is present only in some members of the genus it can be shown between parentheses. This schema can also be used to indicate some protein functions or amino acid motifs where they differ between viruses (e.g. 2Apro or 2Anpgp or 2AH-box/NC or 2ANTPase). There may be multiple copies of a particular genomic region in the picornavirus genome including repeated copies of one particular region (e.g. three 3B’s in the foot-and-mouth disease virus (FMDV) genome) or different types of a particular region (e.g. two different 2A motifs in Ljungan virus of the genus Parechovirus and three different 2A motifs in the duck hepatitis A virus genome of the genus Avihepatovirus).
Replication of viral RNA occurs in tight association with reorganized cytoplasmic membraneous structures. These complexes termed replication organelles contain proteins derived from the whole of the 2BC-P3 region of the polyprotein, including the polymerase (3Dpol, an RNA chain-elongating enzyme), and 2C (an ATPase containing a nucleotide binding sequence motif). The poliovirus and coxsackievirus 3Cpro component has been shown to be required for binding to the 5′-terminal RNA cloverleaf. The small virus-encoded protein, VPg, acts as a replication primer for both positive- and negative-sense RNA synthesis. Prior to replication two uridine residues are covalently linked to the conserved tyrosine at position 3 in VPg to form VPgpUpUOH via a templating mechanism involving a cis-acting replication element (cre) and the virus 3D polymerase. The cre is a stem loop containing the sequence “AAAC” in the loop and is found at various places in the genome depending on virus species/genus. Many compounds that specifically inhibit replication have been described. Mutants resistant to, or dependent on, drugs have been reported. Genetic recombination, complementation, and phenotypic mixing occur. Defective particles, carrying deletions in the CPs or L, have been produced experimentally but have not been observed in natural virus populations.
Biology
Most picornaviruses for which the natural hosts have been identified are specific for one, or a very few host species [exceptions are foot-and-mouth disease virus (FMDV) and encephalomyocarditis virus (EMCV)]. Members of some species can be grown in cell culture. Resistant host cells (e.g., mouse cells in the case of the primate-specific polioviruses) can often replicate virus RNA (for a single round) by transfection with naked, infectious RNA. Transmission is horizontal, mainly by fecal-oral, fomite or airborne routes. Transmission by arthropod vectors is not known, although EMCV has been isolated from mosquitoes and ticks, and poliovirus from flies; therefore mechanical transmission may be possible.
Infection is generally cytolytic, but persistent infections are common with some species and reported with others. Poliovirus infected cells undergo extensive vacuolation as membranes are reorganized into viral replication complexes. Infection may be accompanied by rapid inhibition of cap-dependent translation of cellular mRNAs (2Apro of poliovirus and Lpro of aphthovirus are each powerful inhibitors), mRNA synthesis, and the cellular secretory pathway (poliovirus 2B and 3A have been implicated).
Antigenicity
Serotypes are classified, depending on genus, by cross-protection, neutralization of infectivity, complement-fixation, specific ELISA using a capture format or immunodiffusion. Some serotypes can be identified using hemagglutination-inhibition. Serotypes have been determined for most members of the enteroviruses, aphthoviruses, cardioviruses, erboviruses and teschoviruses, but are increasingly replaced by genotypes (commonly referred to as ‘types’) in clinical or diagnostic practice. Members of different genera are antigenically unrelated where investigated.
Derivation of names
Picornaviridae: an acronym from poliovirus, insensitivity to ether, coxsackievirus, orphan virus, rhinovirus, ribonucleic acid; also, from the prefix "pico" which designates a very small unit of measurement (equivalent to 10-12) and RNA, so describing very small RNA viruses.
Genus demarcation criteria
Members of a genus would normally be expected to have homologous IRES structures and proteins, and their sequences would cluster on the same branch in phylogenetic trees (monophyly). The members of all known picornavirus genera differ by (i) genome maps that exhibit distinctive features in comparison to their closest relatives, (ii) significant divergence (number of differences per site between sequences) of the orthologous proteins exceeding 66% of P1cap and 64% of 2Chel, 3Cpro and 3Dpol [these values are based on current sequence data and may vary with additional data available in future], (iii) lack of detectable homology of proteins L (if present), 2B, 3A, 3B. If a new virus meets these rules, a novel species or genus may be proposed. The Picornaviridae Study Group recommends that strain designations should include information on host species, lab-specific ID number, country of sampling, year of sampling, e.g. seal/ABC1234/USA/2015. When a new picornavirus species is proposed, a preferred virus name should be provided.
Species demarcation criteria
A picornavirus species is a class of phylogenetically related viruses that would normally be expected to share (i) a significant degree of amino acid identity of P1, 2C, 3C and 3D proteins, (ii) monophyly in phylogenetic trees, (iii) essentially identical genome maps, and (iv) a significant degree of compatibility in proteolytic processing, replication, encapsidation and genetic recombination.
Relationships within the family
The orthologous proteins 1B, 1C, 1D, 2C, 3C and 3D are conserved in all picornaviruses and may be used for sequence alignments between viruses, whereas 1A, 2A, 2B, 3A and 3B are highly divergent among members of different picornavirus genera.
Viruses of each picornavirus genus are phylogenetically distinct from members of other genera in those genome regions which are orthologous, i.e. P1cap (Figure 4A. Picornaviridae), 2Chel, 3Cpro 3Dpol (Figure 4B. Picornaviridae). Divergence between the members of a genus may be as high as 66% for the P1 polyprotein and 64% for the 2C, 3C and 3D proteins. Divergence between the members of different genera is usually higher.
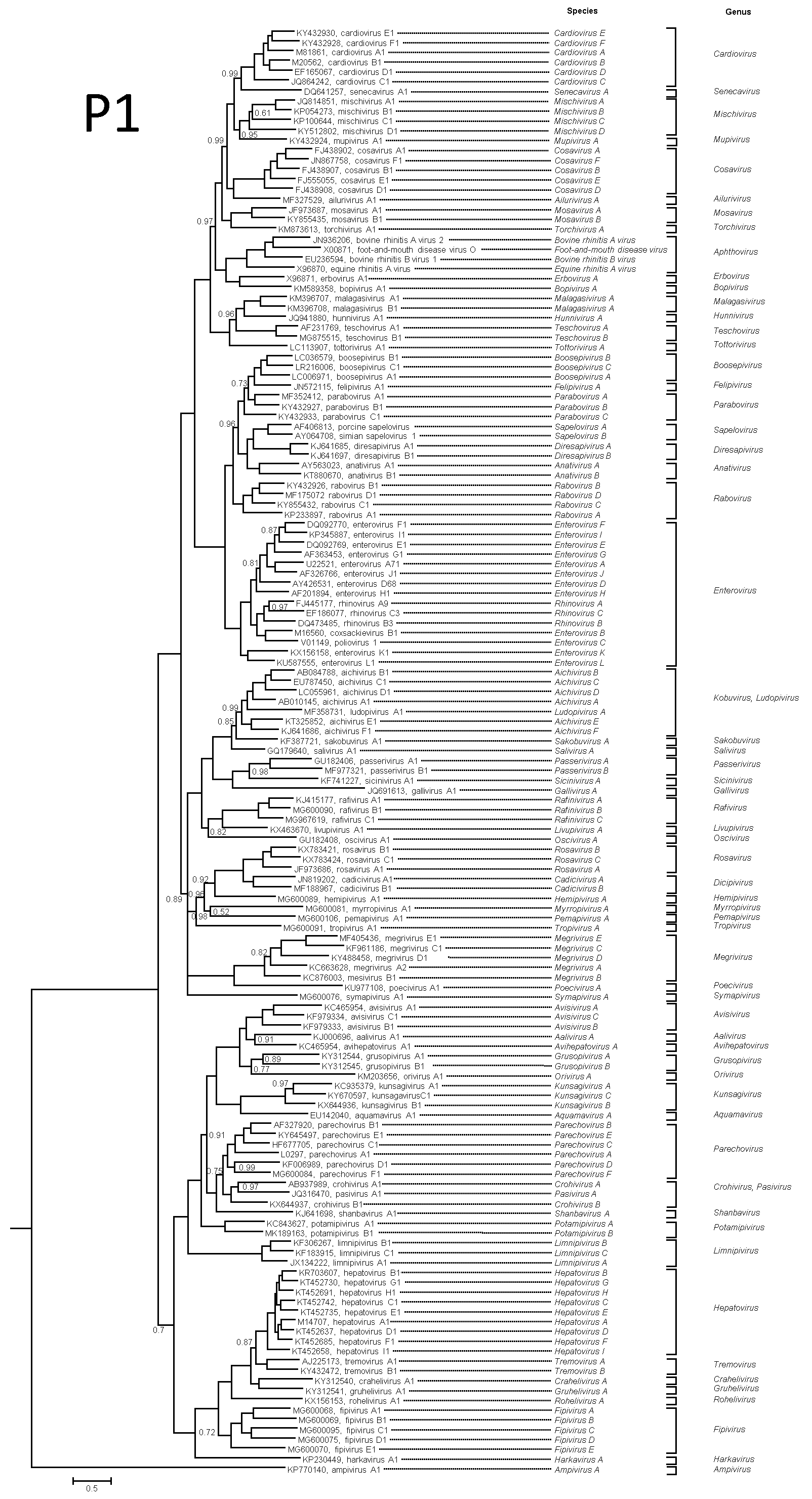 |
| Figure 4A. Picornaviridae. P1 protein phylogenetic tree showing the relationships between the classified members of the family Picornaviridae. For clarity, unclassified picornaviruses were not included. The maximum likelihood tree was produced with MrBayes using the GTR+G+I substitution model. The tree is rooted to the ampivirus outgroup. Tips are labelled with Genbank accession numbers and virus names. The taxonomy (species and genus) is shown to the right. Numbers at nodes indicate posterior probabilities obtained after 3,000,000 generations with values of 1 omitted. This phylogenetic tree and corresponding sequence alignment are available to download from the Resources page. |
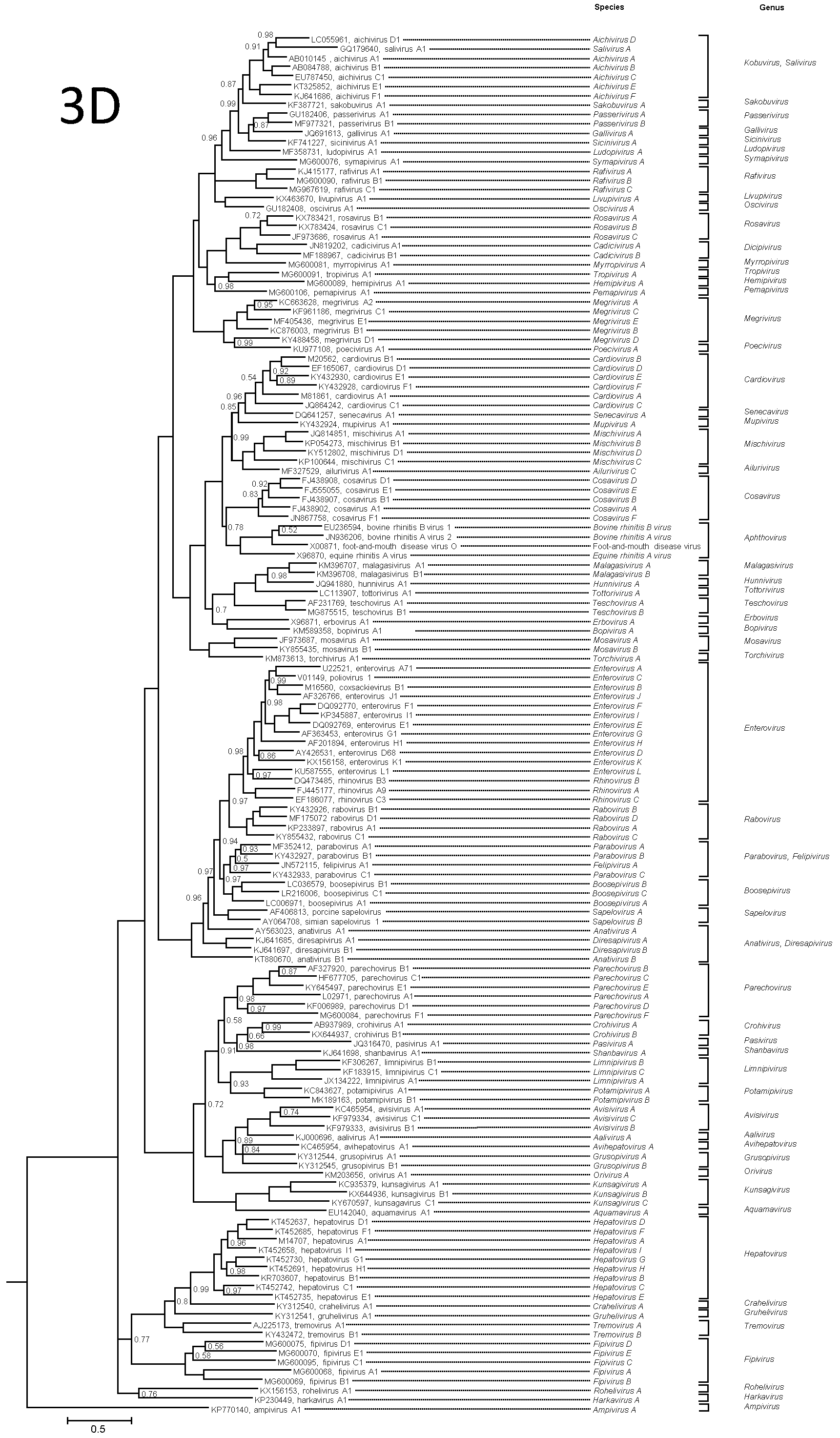 |
| Figure 4B. Picornaviridae. 3Dpol protein phylogenetic tree showing the relationships between the classified members of the family Picornaviridae. For clarity, unclassified picornaviruses were not included. The maximum likelihood tree was produced with MrBayes using the GTR+G+I substitution model. The tree is rooted to the ampivirus outgroup. Tips are labelled with Genbank accession numbers and virus names. The taxonomy (species and genus) is shown to the right. Numbers at nodes indicate posterior probabilities obtained after 2,200,000 generations with values of 1 omitted. This phylogenetic tree and corresponding sequence alignment are available to download from the Resources page. |
Relationships with other taxa
The presence of 1–3 domains with detectable similarity to the rhv-like superfamily with characteristic folding ("β-barrel") in the capsid proteins is common to most members of the order Picornavirales. Likewise, a 'replication block' comprising a RNA-helicase domain (P-loop NTPase), a peptidase C-like proteinase and a RNA-directed RNA polymerase 1 domain (RT-like superfamily) is also present in all members of Picornavirales and many unclassified picorna-like viruses. Capsid proteins and proteins of the replication block show various degree of similarity. The presence of a small genome-linked protein which is also the replication primer is also common to many small positive-sense RNA viruses. A genome organization with a single open reading frame and CP-encoding gene region at the 5′-end and the gene region encoding the replication block proteins at the 3′-end is common to members of the families Picornaviridae and Iflaviridae.
Related, unclassified viruses
| Virus name | Accession number | Virus abbreviation |
| avocet picornavirus A MW13 | MH453807 | |
| avocet picornavirus B MW14 | MH453809 | |
| avocet picornavirus B MW15 | MH453810 | |
| avocet picornavirus B MW16 | MH453808 | |
| bat sapelovirus Bat/CAM/Sap-P24/2013 | KX644938 | |
| Beihai conger picornavirus | MG600065 | |
| Burpengary virus 1 | MK882499 | |
| California sea lion sapelovirus 1 [1162] | JN420368 | |
| California sea lion sapelovirus 2 [1153] | JN420367 | |
| canine sapelovirus [dog/Hong Kong/325F/2008] | JN831356 | |
| Fujian spotted paddle-tail newt picornavirus | MG600085 | |
| grey squirrel picornavirus | MT152345 | |
| Guangdong fish caecilians picornavirus | MG600103 | |
| Guangdong red-banded snake picornavirus | MG600082 | |
| Guangxi changeable lizard picornavirus 1 | MG600104 | |
| Guangxi changeable lizard picornavirus 2 | MG600105 | |
| Guangxi Chinese leopard gecko picornavirus | MG600088 | |
| Hainan black-spectacled toad picornavirus | MG600086 | |
| Ia io picornavirus (sapelo-like) [bat/China/2010] | JQ814852 | |
| marmot sapelovirus 2 [HT6/China/2013] | KY855433 | MmSV-2 |
| pingu picornavirus | MH255796 | |
| Rhinolophus ferrumequinum picornavirus [BtRf-PicoV-2/YN2012] | KJ641684 | RfPV-2 |
| Rhinolophus picornavirus Guizhou-Rr100 | MF352415 | |
| Rhinolophus picornavirus Henan-#Rf265 | MF352416 | |
| sapelo-like bat picornavirus [NC16A] | HQ595340 | |
| sapelo-like bat picornavirus [LMH22A] | HQ595341 | |
| sapelo-like bat picornavirus [MH9F] | HQ595342 | |
| sapelo-like bat picornavirus [SK17F] | HQ595343 | |
| sapelo-like bat picornavirus [TLC5F] | HQ595344 | |
| sapelo-like bat picornavirus [TLC21F] | HQ595345 | |
| sapelo-like bat picornavirus [BtMr-PicoV/JX2010] | KJ641686 | |
| sapelo-like bat picornavirus [BtMf-PicoV-2/GD2012] | KJ641691 | |
| sapelo-like bat picornavirus [BtRs-PicoV/GD2012] | KJ641695 | |
| sapelo-like bat picornavirus [bat/Henan-Mr221/China/2012] | MF352431 | |
| sapelo-like bat picornavirus [BtRh-PicoV/SC2013] | KJ641693 | |
| sapelo-like bat picornavirus [BtMf-PicoV-2/SAX2011] | KJ641699 | |
| sapelo-like bat picornavirus [BtMf-PicoV/FJ2012] | KJ641687 | |
| sapelo-like bat picornavirus [BtMf-PicoV-1/GD2012] | KJ641690 | |
| sapelo-like bat picornavirus [BtVs-PicoV/SC2013] | KJ641696 | |
| sapelo-like bat picornavirus [BtMa-PicoV/FJ2012] | KJ641689 | |
| sapelo-like bat picornavirus [BtRs-PicoV/YN2010] | KJ641694 | |
| sapelo-like bat picornavirus [CAM/Sap-P24/2013] | KX644938 | |
| sapelo-like pigeon picornavirus A [pigeon/Norway/03/603-7/2003] | FR727145 | |
| sapelo-like pigeon picornavirus B [pigeon/Norway/03/641/ 2003] | FR727144 | |
| sapelo-like pigeon picornavirus B [pigeon/GAL-7/2010/Hungary] | KC560801 | |
| sapelo-like porcine picornavirus Japan pig/Isi-Im1/JPN/2015 | LC386162 | |
| Suncus murinus picornavirus Wencheng-Sm294 | MF352410 | |
| Tasmanian devil-associated sapelovirus | MK521918 | |
| Wenling brown-lined puffer picornavirus | MG600100 | |
| Wenling crossorhombus picornavirus | MG600095 | |
| Wenling fish picornavirus 1 | MG600078 | |
| Wenling hoplichthys picornavirus | MG600101 | |
| Wenling lepdotrigla picornavirus | MG600079 | |
| Wenling rattails picornavirus | MG600077 | |
| Wenling scaldfish picornavirus 1 | MG600096 | |
| Wenling scaldfish picornavirus 2 | MG600097 | |
| Wenling sharspine skate picornavirus | MG600093 | |
| Wenling thamnaconus septentrionalis picornavirus | MG600080 | |
| Western African lungfish picornavirus | MG600102 | |
| Wuhan carp picornavirus | MG600066 | |
| Wuhan sharpbelly picornavirus 1 | MG600067 | |
| Yancheng osbecks grenadier anchovy picornavirus | MG600099 | |
| zebrafish picornavirus | MH368041 | |
| Zhejiang banded bullfrog picornavirus | MG600087 |
Virus names and virus abbreviations are not official ICTV designations.

