Subviral Agent: Viroids
Francesco Di Serio, Robert A. Owens, Shi-Fang Li, Jaroslav Matoušek, Vicente Pallás, John W. Randles, Teruo Sano, Jacobus Th. J. Verhoeven, Georgios Vidalakis, and Ricardo Flores
Corresponding author: Francesco Di Serio (francesco.diserio@ipsp.cnr.it)
Edited by: F. Murilo Zerbini and Sead Sabanadzovic
Posted: November 2020
PDF: ICTV_Viroids.pdf
Summary
Viroids are small (a few hundred nucleotides long), circular, single-stranded, non-protein-coding RNAs that replicate autonomously in higher plants. Some are pathogenic, but others infect their hosts without causing any obvious symptoms. Unlike the genomes of the majority of viruses, viroid genomes are not protected by a protein capsid (Table 1.Viroids).
Table 1.Viroids. Characteristics of viroids
| Characteristic | Description |
| Typical member | potato spindle tuber viroid (V01465), species Pospiviroid fusituberis, family Pospiviroidae |
| Genome | Single-stranded, circular RNA of about 250–430 nt adopting rod-like, quasi rod-like or branched conformations of minimum free energy with conserved structural motifs. Viroid RNAs do not code for proteins but contain the information to manipulate the transcription, processing and trafficking machineries of their host plants to invade them systemically |
| Replication | Mediated by a host DNA-dependent RNA polymerase, redirected to recognize RNA templates, through a symmetric (family Avsunviroidae) or asymmetric (family Pospiviroidae) RNA-RNA rolling-circle mechanism occurring in chloroplasts or nuclei, respectively. RNA strands of both polarities accumulate in infected tissues, with the (+) polarity assigned by convention to the strand reaching the higher level in vivo. The oligomeric (+) strands are cleaved by a type-III RNase and circularized by DNA ligase 1 redirected to act on RNA substrates (Pospiviroidae), or the oligomeric (+) and (-) strands self-cleave through embedded hammerhead ribozymes and then are ligated by a chloroplastic tRNA ligase (Avsunviroidae). |
| Translation | Absent |
| Host range | Plants (dicotyledons and some monocotyledons) |
| Taxonomy | Two families: Avsunviroidae and Pospiviroidae including 8 genera and 45 species |
Physicochemical and physical properties
Viroid molecules, with a Mr of 80–125×103 (Diener 1971), display extensive internal base pairing and assume compact conformations in vitro (Sanger et al., 1976, Gross et al., 1978) and in vivo (López-Carrasco and Flores 2017). The most frequent is a rod-like or quasi-rod-like conformation of about 50 nm in length. These structures denature by cooperative melting (Tm in 10 mM Na+ at about 50 °C) to single-stranded circles about 100 nm in contour length. Viroids can also form metastable structures that contain functionally important hairpins. Several viroids adopt branched conformations and are insoluble in 2 M LiCl, as opposed to those assuming rod-like conformations that are soluble under the same conditions. Some viroids also contain elements of tertiary structure like kissing-loops (Flores et al., 2012, Steger and Perreault 2016, Steger et al., 2017).
Nucleic acid
Viroid RNAs of both polarities are found in infected hosts. By convention, the (+) polarity is assigned to the RNA strand accumulating at higher level in vivo. Circular forms of (+) and (-) polarity are generated in tissues infected by some viroids (family Avsunviroidae), while in others (family Pospivioidae) only the (+) polarity strand generates the circular form. Genome sequences of viroids vary from about 250 to 430 nt and are rich in G+C (53–60%), with the exception of avocado sunblotch viroid (ASBVd), which has 38% G+C.
Genome organization and replication
There is no evidence that viroid genomes encode proteins. Instead, viroid RNAs contain structural motifs that are associated with functional roles (Flores et al., 2012, Steger and Perreault 2016, Steger et al., 2017, Ding 2010). These structural elements, conserved among members of several species and genera, allow viroids to use host pathways to replicate and move systemically within plants. Some viroid genomes (family Avsunviroidae) contain cis-acting hammerhead ribozymes embedded in the strands of both polarities that catalyze RNA self-cleavage during replication (probably assisted by host proteins). Unlike viruses that primarily parasitize the host translation machinery, viroids mainly parasitize host transcription by subverting either nuclear RNA polymerase II (family Pospiviroidae) or a nuclear-encoded chloroplastic RNA polymerase (family Avsunviroidae) to recognize RNA templates. The oligomeric RNAs identified in infected tissue are most likely the replicative intermediates generated by a rolling-circle mechanism with two variants (asymmetric and symmetric) and three steps (RNA polymerization, cleavage and ligation). Initiation of RNA synthesis for potato spindle tuber viroid (PSTVd) and ASBVd appears to occur at specific sites, suggesting that it is promoter-driven. In chloroplast-replicating viroids (family Avsunviroidae) the oligomeric RNAs self-cleave in vitro and in vivo through hammerhead structures to produce unit-length strands, but in nuclear-replicating viroids (family Pospiviroidae) cleavage is catalyzed by host enzymes of the RNase III class (Figure 1.Viroids). Ligation of PSTVd, and possibly of all nuclear-replicating viroids, is mediated by nuclear DNA ligase 1, redirected to recognize RNA substrates, whereas this step is catalyzed by a chloroplastic tRNA ligase for chloroplast-replicating viroids (family Avsunviroidae) (Flores et al., 2014). DNA-dependent RNA polymerases introduce mutations while transcribing viroid RNAs during replication. Therefore, infected plants often contain a heterogeneous population of viroid molecules composed of a spectrum of closely related variants generally showing more than 90% sequence similarity, the typical features of a quasispecies (Codoñer et al., 2006).
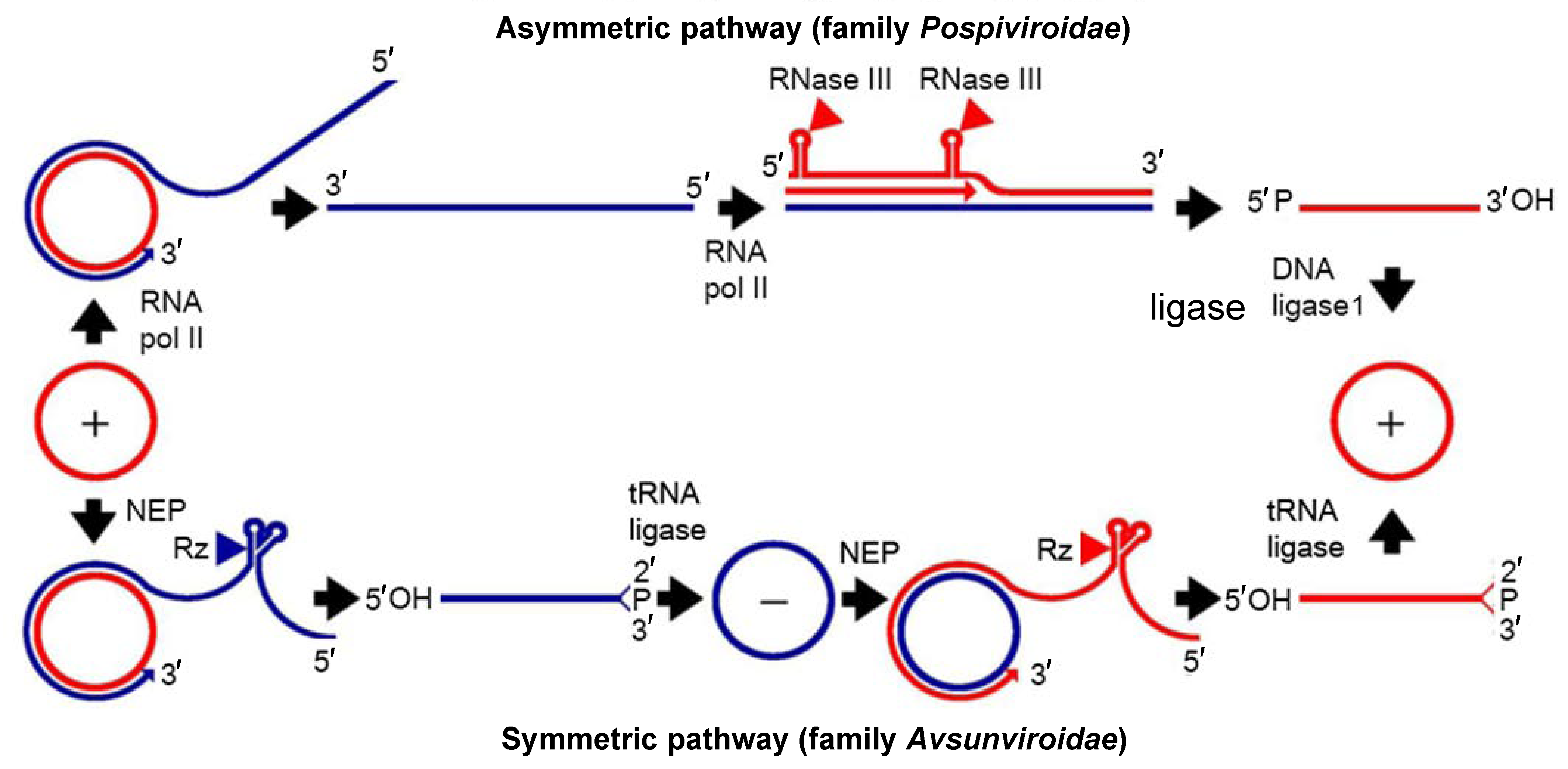 |
| Figure 1.Viroids. Models for viroid replication. The plus polarity (red lines) is assigned by convention to the most abundant infectious RNA and the minus polarity (blue lines) to its complementary strand. The alternative asymmetric and symmetric pathways involve one and two rolling circles, respectively. In the symmetric variant (family Avsunviroidae), transcription is carried out by the nuclear-encoded chloroplastic RNA polymerase (NEP), and cleavage of plus and minus multimeric strands is mediated by hammerhead ribozymes (Rz), which lead to linear monomeric RNAs with 5′-hydroxyl and 2′-3′-cyclic phosphodiester termini that are ligated by a chloroplastic tRNA ligase. Arrowheads denote the cleavage sites. In the asymmetric variant (family Pospiviroidae), the RNA polymerase II (RNA pol II) transcribes the viroid RNAs, cleavage of plus multimeric strands relies on a host factor, likely an RNase III, which generates linear monomeric RNA containing 5′-phosphomonoester and 3′-hydroxyl termini that are ligated by the DNA ligase 1. Adapted with modifications from (Flores et al., 2009). |
Biology
The main biological features of viroids are their ability to replicate autonomously and infect their host plants systemically. These features discriminate viroids from viroid-like satellite RNAs, which are small circular non-protein-coding RNAs whose infectivity strictly depends on a co-infecting helper virus (Navarro et al., 2017); this aspect needs to be carefully ascertained before classifying a small circular RNA as a viroid. Autonomous replication and systemic movement must be experimentally proven by inoculating the candidate viroid RNA into virus-free host(s) followed by recovery of viroid RNA from the systemically invaded tissues of the inoculated plant (Di Serio et al., 2018). The time needed for the systemic invasion of plants (generally weeks or months) depends on the viroid/host combination. Experimental transmission to herbaceous hosts in the greenhouse is achieved by rubbing carborundum-dusted leaves with crude extracts or preparations of total nucleic acids extracted from infected tissue; alternatively, plasmid DNAs containing dimeric head-to-tail cDNA inserts or their in vitro RNA transcripts may be used. The same inoculum can be used to imbibe a sterile razor or a fine needle and slash-inoculate woody hosts or make a puncture in young stems of herbaceous hosts, respectively (Duran-Vila et al., 1988, Ambrós et al., 1998, Steyn et al., 2016). Biolistic approaches (Matousek et al., 2004, Matousek et al., 2007) and agroinoculation are also effective in transmitting viroids (Gardner et al., 1986, Salazar et al., 1988, Marquez-Molins et al., 2019). High pressure injection of nucleic acid preparations effectively transmitted coconut cadang-cadang viroid (CCCVd) to palms (Randles et al., 1977).
Host range
Viroids have been identified only in higher plants (both dicotyledons and monocotyledons). Individual viroids may have either wide or restricted natural host ranges. Some viroids, especially those replicating and accumulating in the chloroplast (family Avsunviroidae), infect only one or a few related plant species (Škorić 2017).
Symptoms
Viroids may cause plant diseases characterized by symptoms on leaves (chlorosis, mosaic, curling, necrosis), flowers (color streaks, flowering delay or cessation), fruits (discoloration, deformation, suture cracking), tubers (size reduction, deformation), bark (canker, distortion, pitting, cracking, scaling, gumming) and/or developmental disorders (stunting, impairment of rooting). To demonstrate the pathogenicity of a viroid requires the fulfillment of Koch’s postulates (Di Serio et al., 2018). Molecular determinants of pathogenesis have been identified in viroid RNAs (Schnölzer et al., 1985, de la Peña et al., 1999), and viroid infection triggers RNA silencing. This mechanism, which regulates host gene expression in eukaryotes, also acts to defend the host against invading nucleic acids (Csorba et al., 2015). Although RNA silencing has been implicated in the pathogenesis of certain viroids (Wang et al., 2004, Dadami et al., 2017), additional mechanisms (still undetermined but possibly related to hormone imbalance and/or activation of host protein kinases) may be responsible for the appearance of macroscopic symptoms elicited by viroids (Flores et al., 2015). Regarding the primary lesion in viroid pathogenicity, analyses of symptom expression and host pathways support the notion that chloroplast-replicating viroids initiate disease (distinctive chloroses) by silencing mRNAs coding for proteins that regulate chloroplast development, whereas the situation remains unclear for those replicating in the nucleus (inciting non-specific alterations like stunting) (Flores et al., 2020).
Movement
Early studies showed that the long-distance movement of PSTVd takes place through the phloem (Palukaitis 1987), with the same route assumed to operate for all viroids. This route is also used by most plant viruses. Viroids also move within the cell (to enter and exit the organelles in which they replicate and accumulate) and over short distances from cell to cell via plasmodesmata. Several structural motifs likely to interact with host proteins have been implicated in viroid trafficking (Ding 2010, Pallás and Gómez 2017, Wang et al., 2018).
Transmission
Viroids infecting horticultural species are transmitted mainly by vegetative propagation of infected plants. In addition, viroids may be transmitted mechanically (e.g. by growers working in crops) or by grafting infected material. Transmission by cutting with sap-contaminated tools may occur in the field, especially in woody hosts. In plants propagated via seeds, viroids may be spread through seed and/or pollen. With the exception of tomato planta macho viroid, which has been reported to be efficiently transmitted by aphids (Galindo et al., 1989), viroids are generally not transmitted by vectors in nature (Škorić 2017).
Relationships among viroids
Nuclear-replicating viroids (family Pospiviroidae) have been reported to be phylogenetically related to chloroplast-replicating viroids (family Avsunviroidae) and to viroid-like satellite RNAs (Elena et al., 1991, Elena et al., 2001). The apparent monophyletic origin of these infectious circular RNAs has been questioned (Jenkins et al., 2000), and more recent theoretical studies indicate that an independent origin for nuclear- and chloroplast-replicating viroids cannot be discarded (Catalán et al., 2019). Several features of viroids support their possible origin in the RNA world (Flores et al., 2014, Diener 1989), including their small size, high G+C content (which would increase the fidelity of primordial RNA polymerases), circularity (which would eliminate the need for a specific initiation site during replication), the absence of protein-coding capacity and the presence in some of them of ribozymes (typical features of an RNA-based world).
Relationships with other taxa
Viroids share their small genome size, circularity and compact secondary structure with viroid-like satellite RNAs that, akin to some viroids, also contain hammerhead ribozymes. However, infectivity of viroid-like satellite RNAs, sometimes named virusoids (Symons and Randles 1999), is strictly dependent upon a co-infecting helper virus. Bioassays showing autonomous replication are needed to conclusively classify a small circular RNA as a viroid (Di Serio et al., 2018).
Family demarcation criteria
Viroids are classified into the families Avsunviroidae and Pospiviroidae on the basis of their biological, biochemical and structural features. Members of the family Avsunviroidae can form, in the strands of both polarities, hammerhead ribozymes that mediate replication in chloroplasts, in which these viroids accumulate. Members of the family Pospiviroidae lack hammerhead ribozymes but contain a central conserved region (CCR) in their rod-like or quasi rod-like conformation and replicate in the nucleus, wherein these viroids also accumulate.

