Family: Virgaviridae
Genus: Tobravirus
Distinguishing features
Tobraviruses have a bipartite genome, a “30K”-like cell-to-cell movement protein and are transmitted by trichodorid nematodes.
Virion
Morphology
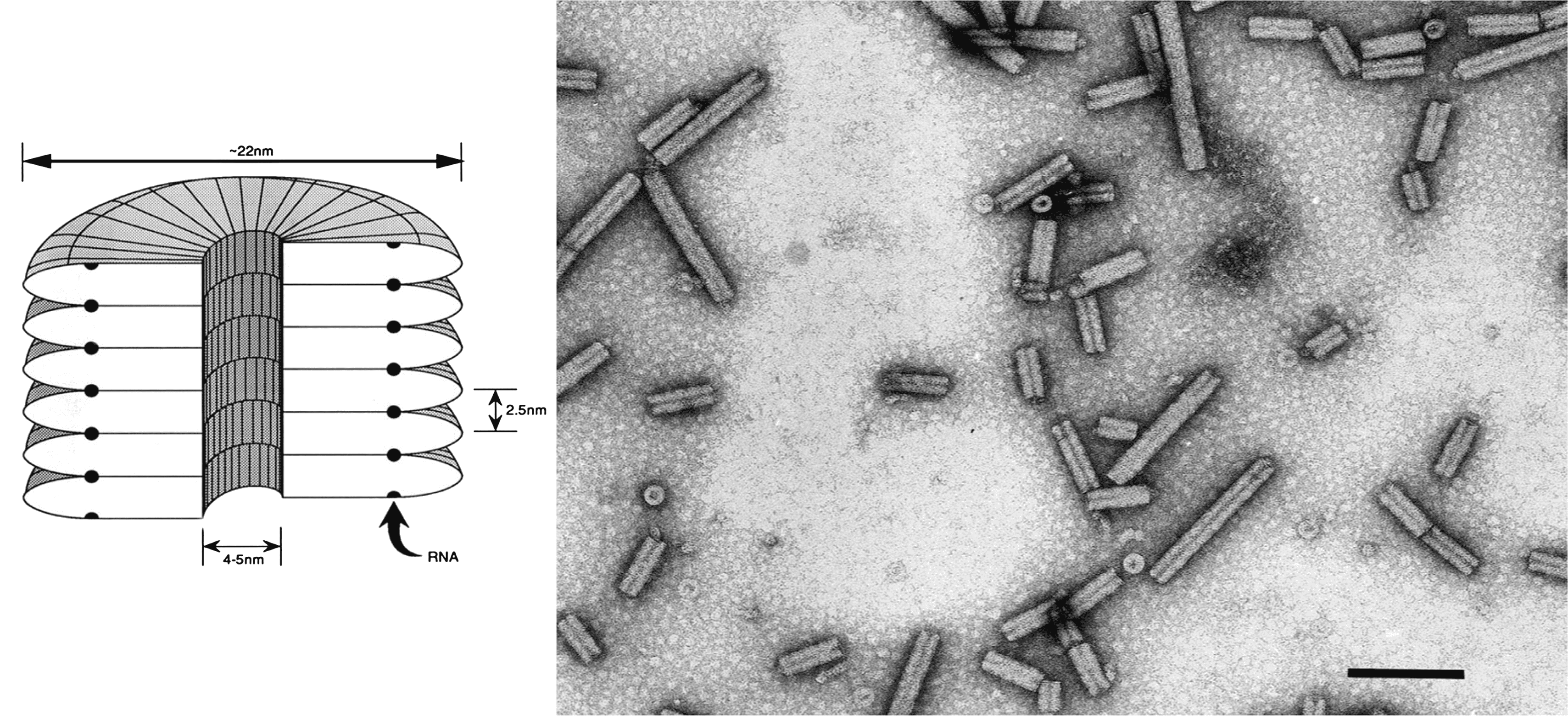
Virions are tubular particles with no envelope (Figure 1. Tobravirus). They are of two predominant lengths, (L) 180–215 nm and (S) ranging from 46 to 115 nm, depending on the isolate. Many strains produce in addition small amounts of shorter particles (Harrison and Robinson 1978, Blanch et al., 2001). The particle diameter is 21.3–23.1 nm by electron microscopy or 20.5–22.5 nm by X-ray diffraction, and there is a central canal 4–5 nm in diameter. Virions have helical symmetry with a pitch of 2.5 nm; the number of subunits per turn has been variously estimated as 25 or 32.
|
Figure 1. Tobravirus. (Left) Diagram of a virion of tobacco rattle virus (TRV), in section. (Right) Negative contrast electron micrograph of particles of TRV. The bar represents 100 nm. |
Physicochemical and physical properties
Virion Mr is 48–50×106 (L particles) and 11–29×106 (S particles). Buoyant density in CsCl is 1.306–1.324 g cm−3. S20,W is 286–306S (L particles) and 155–245S (S particles). Virions are stable over a wide range of pH and ionic conditions and are resistant to many organic solvents, but are sensitive to treatment with EDTA. In N. clevelandii sap, the thermal inactivation point (10 min) of M-type isolates is 80–85 °C.
Nucleic acid
The genome consists of two molecules of linear positive sense ssRNA; RNA 1 is about 6.8 kb and RNA 2 ranges from 1.8 kb to about 4.5 kb in size (varying in different isolates). The 5′ terminus is capped with the structure m7G5′ppp5′Ap. The 3′ terminus can adopt a tRNA-like structure that can be adenylated but not aminoacylated (MacFarlane 1999).
Proteins
Virions contain a single structural protein of 22–24 kDa.
Genome organization and replication
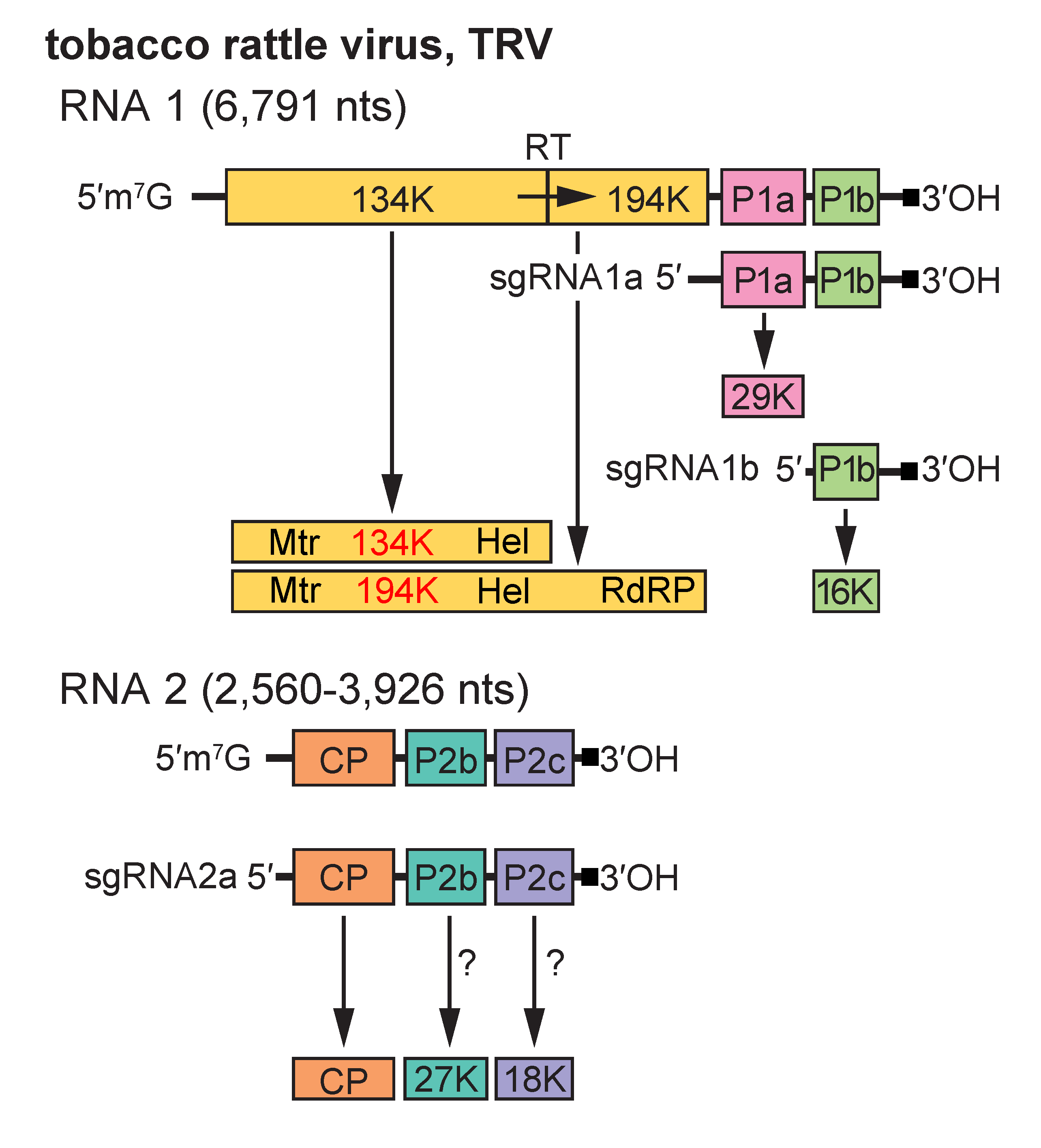
RNA 1 codes for four non-structural proteins: a 134-141 kDa protein terminated by an opal stop codon and a 194-201 kDa protein produced by readthrough of this stop codon, both of which are probably involved in RNA replication; a 29-30 kDa protein (P1a) involved in intercellular transport of the virus; and a 12-16 kDa protein (P1b), which is a suppressor of RNA silencing (Martin-Hernandez and Baulcombe 2008) and determinant of seed transmission, as shown for Pea early-browning virus (PEBV) in pea(Wang et al., 1997). In addition to the virion structural protein, RNA 2 codes for two non-structural proteins, P2b and P2c. The size of P2b ranges from 27 to 40 kDa in different isolates, and that of P2c from 18 to 33 kDa. P2b is absolutely required for transmission by nematodes, whereas mutation of the P2c ORF affects nematode transmission in some strains but not in others (MacFarlane et al., 1996, Vassilakos et al., 2001). The ORFs for P2b and P2c are missing from some laboratory strains that have been maintained by mechanical transmission. RNA 2 of some tobravirus isolates contains an additional small ORF between the CP and P2b ORFs, which codes for a potential 9 kDa protein. RNA2 of TRV isolate SYM has an unusual genomic organisation, with additional, novel ORFs being located upstream of the CP ORF.
RNA 1 is capable of independent replication and systemic spread in plants. The 134–141 kDa and 194–201 kDa replication proteins are translated directly from it, whereas P1a and P1b are translated from sgRNA species 1a and 1b, respectively. RNA 2 does not itself have messenger activity; the CP is translated from sgRNA 2a (Harrison and Robinson 1978, MacFarlane 1999). The means by which the other RNA 2 encoded proteins are expressed is not known but probably also involves sgRNAs (Figure 2. Tobravirus). There is sequence similarity between RNA 1 and RNA 2 at both ends, but the extent of the similarity varies between strains. In some strains, the similar region at the 3′ end is large enough to include some or all of the P1a and P1b ORFs of RNA 1, but it is not known if these ORFs are expressed from RNA 2. Accumulation of virus particles is sensitive to cycloheximide but not to chloramphenicol, suggesting that only cytoplasmic ribosomes are involved in viral protein synthesis. Virions accumulate in the cytoplasm. L particles of pepper ringspot virus (PepRSV) become radially arranged around mitochondria, which are often distorted, and in cells infected with some TRV isolates, “X-bodies” largely composed of abnormal mitochondria and containing small aggregates of virus particles may be produced.
|
Figure 2. Tobravirus. Genome organization and strategy of expression of tobacco rattle virus (TRV). The tRNA structure motifs at the 3′-ends of the RNAs are represented by a dark square. The arrow shows translational readthrough (RT) to produce the larger replication protein. P1a and P1b are translated from separate 3’-terminal subgenomic (sg) mRNAs, sgRNA1a and sgRNA1b. The CP is translated from sgRNA2a; the mechanism by which the P2b and P2c proteins are expressed is unknown. |
Biology
The host ranges are wide, including members of more than 50 monocotyledonous and dicotyledonous plant families. The natural vectors are nematodes in the genera Trichodorus and Paratrichodorus (Trichodoridae); different species being specific for particular virus strains (Ploeg et al., 1991). Adults and juvenile nematodes can transmit, but virus is probably not retained through the molt. Ingested virus particles become attached to the esophageal wall of the nematodes, and are thought to be released by salivary gland secretions and introduced into susceptible root cells during exploratory feeding probes (Macfarlane 2003). Virus can be retained for many months by non-feeding nematodes. There is no evidence for multiplication of virus in the vector and it is probably not transmitted through nematode eggs. The viruses are transmitted through seed of many host species. TRV occurs in Europe (including Russia), Japan, New Zealand and North America; PEBV occurs in Europe and North Africa, and PepRSV occurs in South America. TRV causes diseases in a wide variety of crop plants as well as weeds and other wild plants, including spraing (corky ringspot) and stem mottle in potato, rattle in tobacco, streaky mottle in narcissus and tulip, ringspot in aster, notched leaf in gladiolus, malaria in hyacinth and yellow blotch in sugar beet. PEBV is the cause of diseases in several legumes, including broad bean yellow band, distorting mosaic of bean and pea early-browning. PepRSV causes diseases in artichoke, pepper and tomato.
Most tissues of systemically invaded plants can become infected but in many species virus remains localized at the initial infection site in the roots. In some virus–host combinations, notably TRV in some potato cultivars, limited systemic invasion occurs, and virus may not be passed on to all the vegetative progeny of infected mother plants.
Normal particle-producing isolates (called M-type) are readily transmitted by inoculation with sap and by nematodes (Harrison and Robinson 1978). Other isolates (called nm-type) have only RNA 1, do not produce particles, are transmitted with difficulty by inoculation with sap, and are probably not transmitted by nematodes. nm-type isolates are obtained from M-type isolates by using inocula containing only L particles, and are also found in naturally infected potato plants. They often cause more necrosis in plants than do their parent M-type cultures.
Antigenicity
Tobravirus particles are moderately immunogenic. There is little or no serological relationship between members of the genus, and considerable antigenic heterogeneity among different isolates of the same virus species (Robinson and Harrison 1985).
Derivation of names
Tobra: tobacco rattle virus.
Species demarcation criteria
The criteria demarcating species in the genus are:
- Nucleotide sequences of RNA 1 show <75% identity
- Interspecific pseudo-recombinant isolates cannot be made
- Host ranges differ in specific hosts (e.g. legumes)
- RNA 2 sequences and serological relationships are of limited value