Family: Virgaviridae
Genus: Tobamovirus
Distinguishing features
Tobamoviruses are the only members of the family to have a non-segmented genome. They have a “30K”-like cell-to-cell movement protein, are not vector-transmissible and when seed transmitted, the embryo is not affected. It is the largest genus in the family and the literature is extensive. For reviews of diversity and evolution within the genus see (Gibbs et al., 2015, Lartey et al., 1996, Stobbe et al., 2012).
Virion
Morphology
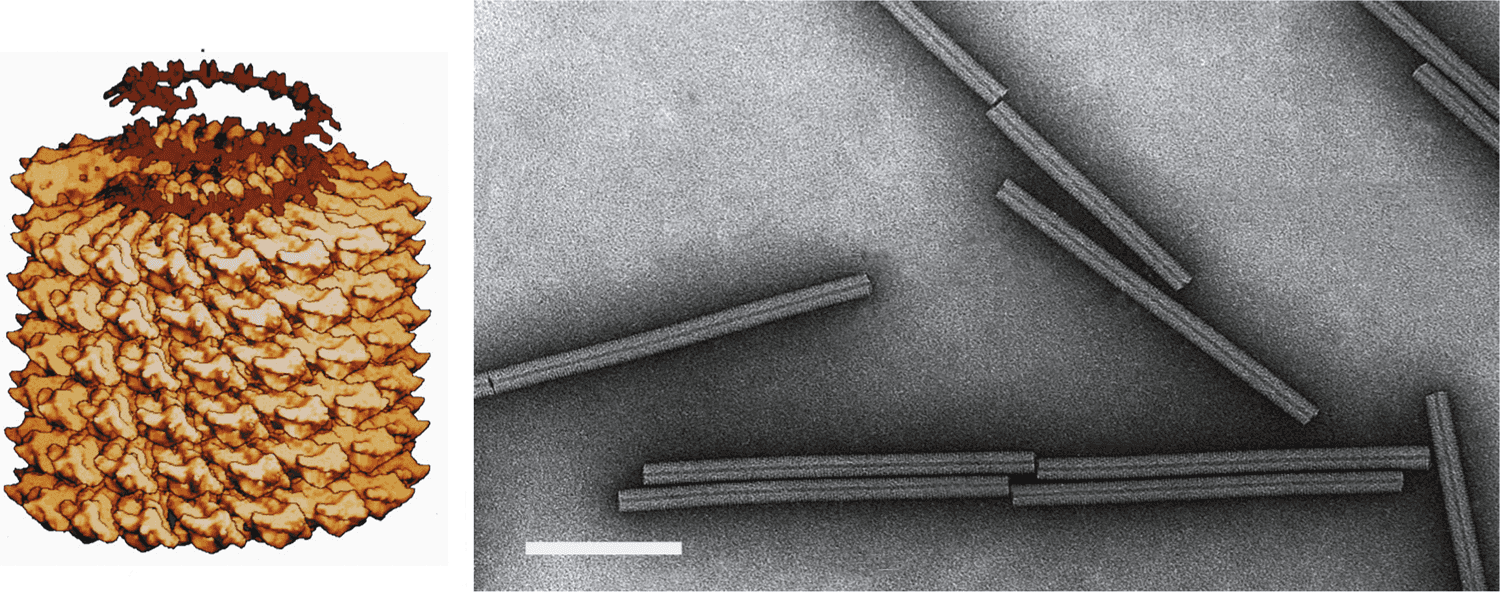
Virions are 18 nm in diameter and have a predominant length of 300–310 nm (Figure 1. Tobamovirus). Structure and assembly of the particles have been reviewed by (Klug 1999). Shorter virions produced by the encapsidation of subgenome-sized RNA are usually a minor component of the virion population, although virions of two species produce an abundant short virion of 32–34 nm. Virions often form large crystalline arrays visible by light microscopy.
|
Figure 1. Tobamovirus. (Left) Model of particle of tobacco mosaic virus (TMV). Also shown is the RNA as it is thought to participate in the assembly process. (Right) Negative contrast electron micrograph of TMV particle stained with uranyl acetate. The bar represents 100 nm. |
Physicochemical and physical properties
Virion Mr is 40×106. Buoyant density in CsCl is 1.325 g cm−3. S20,w is 194S. Tobamoviruses have thermal inactivation points (10 min) of 90 °C and survive in plant sap for many years.
Nucleic acid
The genome is 6.3–6.6 kb in size. An approximately 70 nt long 5′-NTR contains many AAC repeats and few or no G nucleotides. The 0.2–0.4 kb 3′-NTR contains sequences that can be folded into pseudoknots followed by 3′-terminal sequences that can be folded into a tRNA-like, amino acid-accepting structure. The subgenomic mRNAs transcribed in infected cells also have a 5′-terminal cap and 3′-tRNA-like structure. The encapsidation signal is usually located within the ORF encoding the MP (Wilson and McNicol 1995) or within the ORF encoding the CP in the studied isolates of Cucumber green mottle mosaic virus and Sunn hemp mosaic virus.
Proteins
Virions contain a single structural protein (17–18 kDa). Two nonstructural proteins are expressed directly from the genomic RNA: a 124–132 kDa protein terminated by an amber (UAG) stop codon and a 181–189 kDa protein produced by readthrough of this stop codon, both of which are required for efficient replication. A third nonstructural protein (28–31 kDa) is required for cell-to-cell and long-distance movement and belongs to the “30K”-like cell-to-cell movement proteins. The MP is associated with plasmodesmata and has single-stranded nucleic acid binding activity in vitro. The CP is not required for cell-to-cell movement, but has a role in vascular tissue dependent virus accumulation. The replication proteins have also been implicated in virus movement. The MP and CP are expressed from individual 3′-co-terminal subgenomic mRNAs. The MP is expressed early during infection, whereas the CP is expressed later, and at higher levels. The MP and CP are not required for replication in single cells. The N-terminal one-third of the 124–132 kDa protein has similarity with methyltransferase/guanylyl transferases whereas the C-terminal one-third of the 124–132 kDa protein has similarity with RNA helicases (including an NTP-binding motif). The readthrough domain of the 181–189 kDa protein has motifs common to RdRPs.
Genome organization and replication
The single genomic RNA encodes at least four proteins. The 124–132 kDa and 181–189 kDa replication proteins are translated directly from the 5' proximal ORF of the genomic RNA. The 124–132 kDa replication protein contains the Mtr and Hel domains. The 181–189 kDa replication protein additionally contains the polymerase domain, synthesized by occasional readthrough of the leaky termination codon of the 124–132 kDa protein encoding ORF. The 181–189 kDa replication protein is the only protein required for replication in single cells, although the 124–132 kDa replication protein is also required for efficient replication. The downstream ORFs encode the 28–31 kDa MP and 17–18 kDa CP, which are translated from their respective 3′ co-terminal sgRNAs, both of which contain a 5′ cap (Figure 2. Tobamovirus). In the members of some species, the MP ORF overlaps both the 181–189 kDa protein and the CP ORFs, whereas in other species it does not overlap either ORF or overlaps one of the ORFs. An ORF that encodes a cysteine-rich protein is located between the 181-189 kDa and MP ORFs in passion fruit mosaic virus. a tobamovirus isolated from maypop, a plant classified in the order Malpighiales (Stobbe et al., 2012).
|
Figure 2. Tobamovirus. Genome organization of tobacco mosaic virus (TMV). Genomic RNA is capped and is template for expression of the 126 and 183 kDa proteins. The 3′ distal movement protein (MP) and capsid protein (CP) ORFs are expressed from separate 3′ co-terminal sgRNAs. The tRNA structure motif at the 3′-end of the RNA is represented by a dark square. |
RNA replication occurs via several steps: (a) synthesis of viral replication proteins by translation of the genomic RNA; (b) translation-coupled binding of the replication proteins to a 5'-terminal region of the genomic RNA; (c) recruitment of the genomic RNA by replication proteins onto membranes and formation of a complex with host proteins TOM1 and ARL8; (d) synthesis of complementary (negative-strand) RNA in the complex; and (e) synthesis of progeny genomic RNA (Ishibashi and Ishikawa 2016).
Biology
Most species have moderate to wide host ranges under experimental conditions, although in nature host ranges are usually quite narrow. The viruses are found in all parts of host plants. Transmission occurs without the help of vectors by contact between plants and sometimes by seed, although this occurs in the absence of infection of the embryo.
Antigenicity
The virions act as strong immunogens and members of different species are serologically distinct.
Derivation of names
Tobamo: from tobacco mosaic virus.
Species demarcation criteria
Many tobamoviruses that were historically designated as strains of tobacco mosaic virus are now defined as separate species based on nucleotide sequence data.
The criteria demarcating species in the genus are:
- Sequence similarity: more than 90% whole genome nt sequence identity is considered to characterize strains of the same species. Most of the sequenced tobamoviruses of different species have considerably less than 90% sequence identity
- Host range: however many of these viruses have wider and more overlapping host ranges in experimental rather than natural situations
- Antigenic relationships between the CPs
Related, unclassified viruses
|
Virus name |
Accession number |
Virus abbreviation |
|
Abutilon yellow mosaic virus |
AbYMV |
|
|
Hoya chlorotic spot virus |
HCSV |
Virus names and virus abbreviations are not official ICTV designations.
* partial genome