Family: Arteriviridae
Margo A. Brinton, Anastasia A. Gulyaeva, Udeni B.R. Balasuriya, Magda Dunowska, Kay S. Faaberg, Tony Goldberg, Frederick C.C. Leung, Hans J. Nauwynck, Eric J. Snijder, Tomasz Stadejek, and Alexander E. Gorbalenya
The citation for this ICTV Report chapter is the summary published as Brinton et al., (2021):
ICTV Taxonomy Report: Arteriviridae 2021 Journal of General Virology, 2021 102(8):001632.
Corresponding author: Margo A. Brinton ([email protected])
Edited by: Peter Simmonds and Stuart G. Siddell
Posted: June 2021
Acknowledgment: AAG and ASG thank Igor Sidorov and Dmitry Samborskiy for help with the DEmARC analysis of arteriviruses, which was partly supported by the EU Horizon2020 EVAg 653316 grant and LUMC MoBiLe Program to AEG.
Summary
This Chapter summarizes diverse biological and genetic characteristics of arteriviruses in the context of the family taxonomy, which includes six subfamilies, 13 genera, 11 subgenera, and 23 species, as of July 2021 (Table 1. Arteriviridae). The few experimentally characterized members of the family Arteriviridae are viruses with spherical, pleomorphic, enveloped virions that have a median diameter of about 50 to 74 nm and contain a linear, positive-sense RNA, multi-cistron genome of approximately 12.7 to 15.7 kb. Infection of vertebrate hosts is vector-independent and can be asymptomatic or produce overt disease. Some arteriviruses are important veterinary pathogens while others infect particular species of wild rodents or African non-human primates and may be characterized only by genome sequencing. The most divergent member found to date infects a marsupial. The taxa at the four hierarchical ranks of the family are monophyletic clusters that were demarcated with DEmARC software by analysis of evolutionary distances separating five replicative protein domains, 3CLpro, NiRAN, RdRp, ZBD and HEL1. These domains are uniquely conserved in the order Nidovirales and enabled a common framework for the taxonomies of the Arteriviridae and other nidovirus families. Compared to the prior 9th Report, a new non-Latinised binomial nomenclature was adopted for most species that uses names of Greek alphabet letters as part of the genera names.
Table 1. Arteriviridae. Characteristics of members of the family Arteriviridae
| Characteristic | Description |
| Example | equine arteritis virus (X53459), species Alphaarterivirus equid, genus Alphaarterivirus |
| Virion | Pleomorphic but roughly spherical particles of 50 to 74 nm in diameter |
| Genome | Linear, positive-sense RNA of 12.7 to 15.7 kb |
| Replication | Cytoplasmic, viral RNA replication occurs on double membrane vesicles derived from the endoplasmic reticulum (ER), is mediated by the virus ribonucleoprotein transmembrane complex consisting of 12 nonstructural proteins; an antigenome RNA serves as the template for replication; discontinuous transcription produces negative-sense, subgenomic RNAs that are copied into subgenomic mRNAs. Assembled capsids bud into the lumen of the ER and are secreted through the cellular vesicle transport pathway. |
| Translation | Cytoplasmic, from viral capped and poly-adenylated genomic and subgenomic mRNAs. |
| Host range | Vertebrates, predominantly non-human mammals |
| Taxonomy | Realm Riboviria, kingdom Orthornavirae, phylum Pisuviricota, class Pisoniviricetes. The family belongs to the order Nidovirales, suborder Arnidovirineae, and includes six subfamilies (Equarterivirinae, Simarterivirinae, Variarterivirinae, Zealarterivirinae, Heroarterivirinae, and Crocarterivirinae), thirteen genera comprising 23 species, 15 of which are assigned to eleven subgenera. |
Equine arteritis virus (EAV), and porcine reproductive and respiratory syndrome virus type 1 and porcine reproductive and respiratory syndrome virus type 2 (PRRSV-1 and PRRSV-2), which are often collectively referred to as PRRSV in the literature as well as in this chapter (unless specified otherwise), are the best characterized and most frequently sampled arteriviruses. The text below is based mostly on research with these three viruses but also includes characterization of lactate dehydrogenase-elevating virus (LDV), simian haemorrhagic fever virus (SHFV) and wobbly possum disease virus (WPDV).
Virion
Morphology
Virions are pleomorphic but roughly spherical particles. By cryo-electron microscopy, PRRSV-2 particle diameters are estimated to range from 50 to 74 nm, with a median value of 54 nm, but with a few particles larger than 60 nm (Figure 1. Arteriviridae). The genomic RNA is surrounded by the nucleocapsid (N) protein that forms a roughly spherical nucleocapsid lacking icosahedral symmetry of about 39 nm in diameter. The nucleocapsid is surrounded by a lipid envelope with small surface projections consisting of the major viral envelope protein GP5 (glycoprotein 5) and an associated M (membrane) protein covering the entire virion surface (Spilman et al., 2009). Virions also have complexes of the minor envelope proteins on their surface.
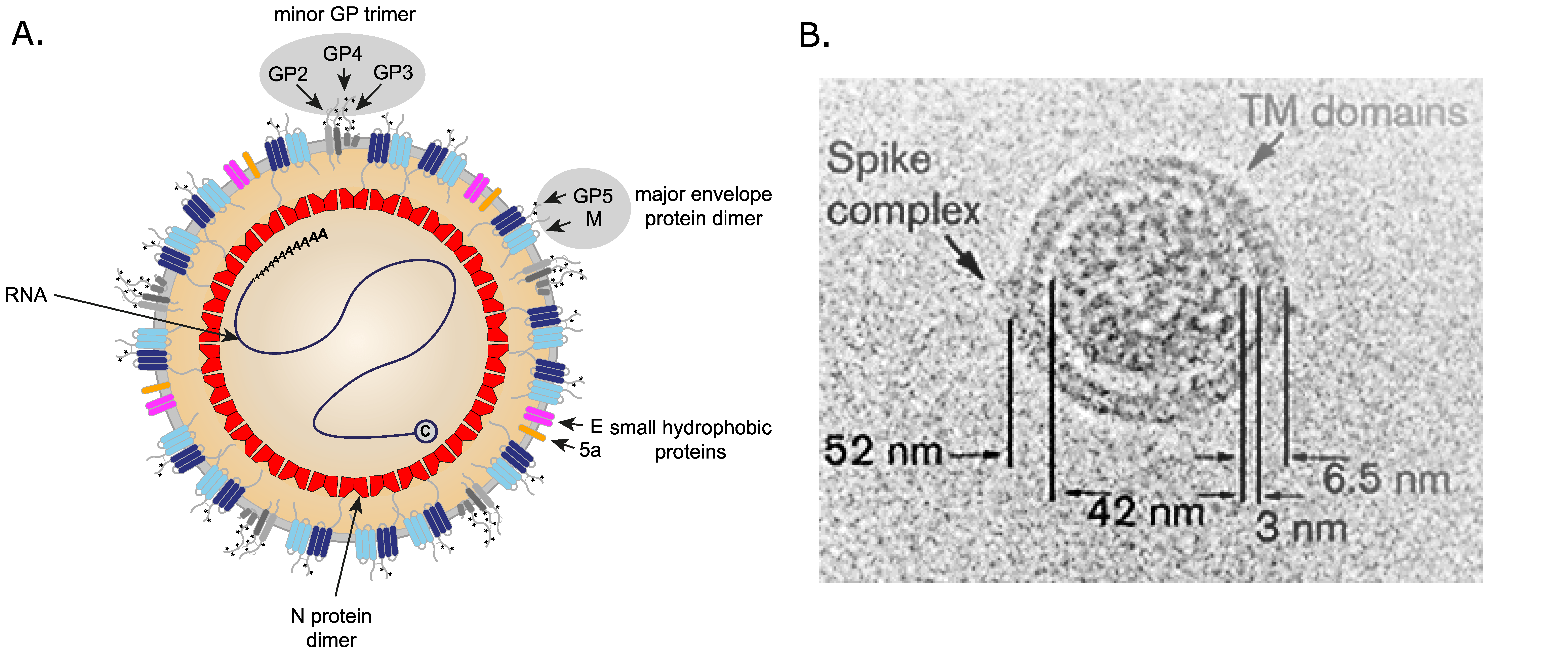 |
| Figure 1. Arteriviridae. Arterivirus virion structure. (A) Diagram of an arterivirus particle with the locations of the structural proteins indicated. (B) Cryo-EM image of a representative porcine reproductive and respiratory syndrome virus 2 particle. A (putative) spike protein complex and striations in the virion envelope that may be envelope transmembrane domains are indicated. Adapted from (Spilman et al., 2009). |
Physicochemical and physical properties
The buoyant density of arterivirus particles is estimated to be 1.13 to 1.17 g cm−3 in sucrose. Reported sedimentation coefficients for arterivirus particles range from 200S to 300S. Virions are stable when stored at −70 °C, but their half-life progressively decreases with increasing temperature or pH outside the range of 6.0−7.5. Arteriviruses are also inactivated by lipid solvents such as ether, butanol and chloroform, and are extremely sensitive to detergent treatment. A brief incubation with a nonionic detergent such as 0.01% NP40 or Triton X-100 efficiently disrupts the viral envelope.
Nucleic acid
Virions contain a single molecule of linear, positive-sense, genomic RNA that ranges in length from about 12 to 16 kb (Figure 2. Arteriviridae). The infectious genomic RNA contains a 5′-type I cap structure and a 3′-terminal poly(A) tract flanking multiple and mostly overlapping ORFs. The two most 5′-terminal and largest ORFs, known as ORF1a and ORF1b, encode nonstructural proteins (nsp) derived from polyproteins, while the 3′-ORFs encode structural virion proteins.
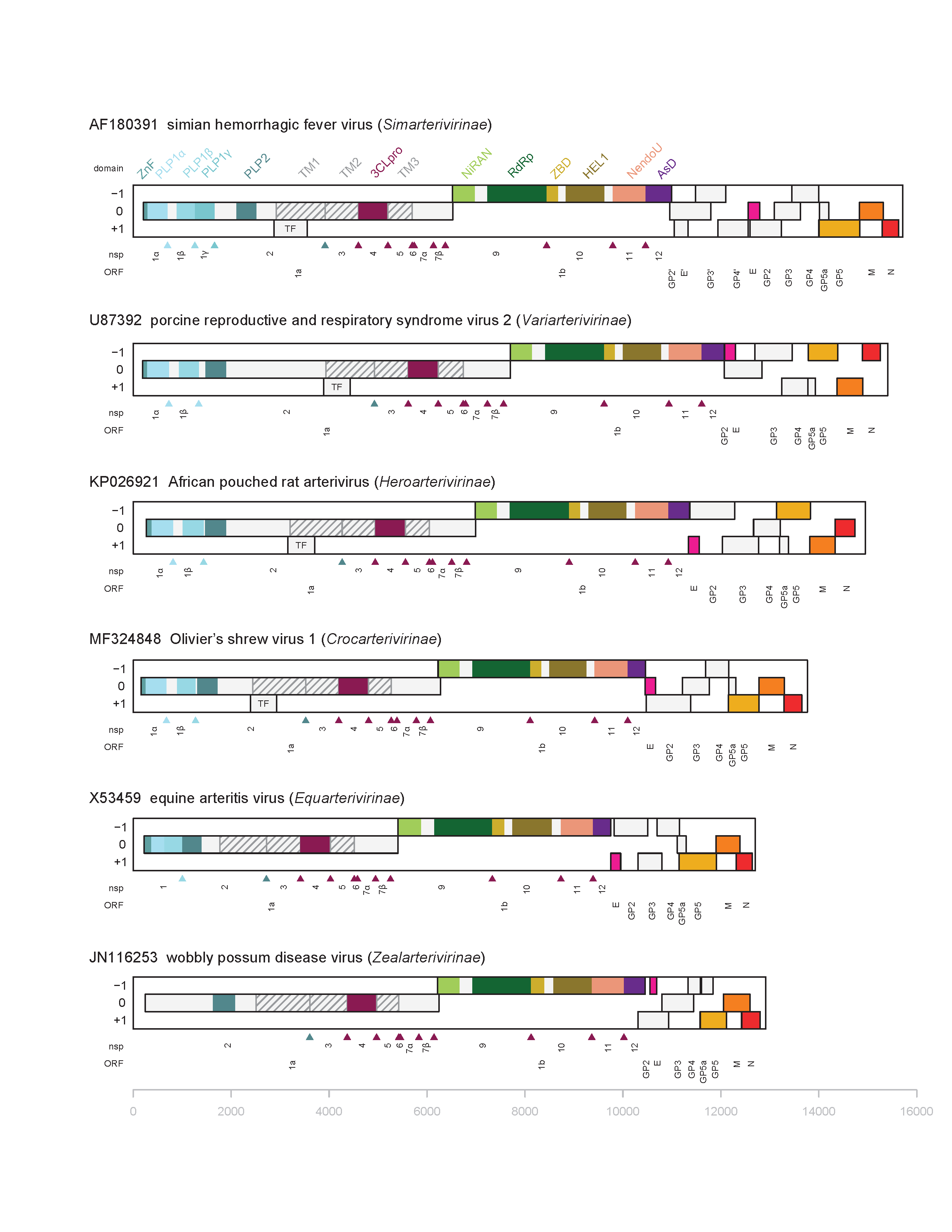 |
| Figure 2. . Arteriviridae. Genome organization and replicase protein domains of arteriviruses representing six subfamilies. Open reading frames (ORFs) are depicted as rectangles on respective reading frame (RF) in genomes of six arteriviruses representing different subfamilies and labelled below the genome; the 5′-terminal and largest ORFs are ORF1a and ORF1b that together with the TF ORF in some arteriviruses encode components of the replicase; the 3′-ORFs encode structural proteins (see Table 2. Arteriviridae). Replicase nsps produced from ORF1a, TF and ORF1b are indicated below the genome and above the respective ORFs (see Table 3.Arteriviridae for nsp2TF and nsp2N, and pp1a-derived nsp8 which are not depicted), and replicase domains are listed above genome. ZnF, Zn-finger; PLP1a, PLP1b, PLP1g, and PLP2 are various papain-like proteases; TM1, TM2 and TM3, three transmembrane domains; TM1 includes a Cys-rich C-terminal domain; 3CLpro, 3C-like protease; NiRAN, nidovirus RdRp-associated nucleotidyltransferase; RNA-directed RNA polymerase (RdRp); ZBD, Zn-binding domain; HEL1, superfamily 1 helicase; NendoU, nidovirus uridylate-specific endonuclease; AsD, arterivirus-specific domain. The GenBank and RefSeq records of genome sequences and primary literature were used to depict genome and protein organization of the respective viruses. |
Proteins
Seven structural proteins have been identified in EAV, PRRSV-1 and PRRSV-2 virions by diverse analyses (Table 2.Arteriviridae). The nucleocapsid (N) proteins of EAV and PRRSV-2 have been shown to dimerize. Crystal structures of the putative dimerization domain of N protein has been determined for PRRSV-2 and EAV N (Protein Data Bank IDs 1P65 and 2I9F, respectively). Based on protein homology, the virions of all arteriviruses contain two major (GP5 and M) and five minor (E, GP2, GP3, GP4 and GP5a) envelope proteins (Figure 1. Arteriviridae). The major glycoprotein, GP5, spans the membrane three times and forms a disulfide-linked heterodimer with the triple membrane spanning M protein. The formation of this heterodimer by conserved cysteine residues is essential for virus infectivity and GP5 antigenicity (Faaberg et al., 1995). The GP5 proteins of EAV and SHFV are predicted to possess 98 residues on the outside of the virion, while the GP5 proteins of PRRSV and LDV are predicted to have ectodomains of approximately 30 amino acids. GP5a is a small membrane protein that is incorporated into arterivirus virions (Johnson et al., 2011, Firth et al., 2011). The E proteins of EAV and PRRSV are fatty acid acylated but E protein myristoylation was shown not to be essential for the infectivity of either virus. The E protein appears to be an ion-channel protein that may function to facilitate virion uncoating during cell entry. The synthesis of the SHFV E and GP2 is directed by separate mRNAs but for other arteriviruses these proteins are thought to be produced by a bicistronic subgenomic mRNA2a/b.
The LDV GP3 is a soluble, highly glycosylated, non-structural protein (Faaberg and Plagemann 1997). GP2, GP3 and GP4 form heterotrimers on the surface of the virus particle (EAV and PRRSV). The orthologous proteins encoded by the respective LDV ORFs, known as 2a/b, 3 and 4, have not been confirmed as structural components of LDV, nor has the trimerization of these proteins been assessed. The SHFV genome encodes a cluster of four additional 3′-proximal ORFs (2b′, 2a′, 3′ and 4′) that encode E′, GP2′, GP3′ and GP4′, which may have evolved from a duplication of the ORFs 2 a/b to 4 (Table 2. Arteriviridae and Figure 2. Arteriviridae). Each of the eight minor proteins encoded in the 3′-region of the SHFV genome were shown to be required for the production of infectious virus (Vatter et al., 2014). A soluble, non-virion associated form of the ORF3-encoded glycoprotein (GP3) is released from cells infected with LDV and PRRSV-2.
Table 2. Arteriviridae. Virion-associated proteins of simian haemorrhagic fever virus
| Protein | Amino acids | ORF | (Putative) Function(s) |
|---|---|---|---|
| Ec | 80 | 2a | Small myristylated integral envelope protein, postulated ion channel protein |
| GP2 | 214 | 2b | Minor glycoprotein, part of GP2/GP3/GP4 heterotrimer |
| GP3 | 179 | 3 | Minor glycoprotein, part of GP2/GP3/GP4 heterotrimer |
| GP4 | 205 | 4 | Minor glycoprotein, part of GP2/GP3/GP4 heterotrimer |
| E′ | 94 | 2b′ | Additional minor protein of Simarterivirinae members |
| GP2′ | 281 | 2a′ | Additional minor glycoprotein of Simarterivirinae members |
| GP3′ | 204 | 3′ | Additional minor glycoprotein of Simarterivirinae members |
| GP4′ | 182 | 4′ | Additional minor glycoprotein of Simarterivirinae members |
| GP5 | 278
| 5
| Major glycoprotein, carries the main determinants for neutralization, forms heterodimer with M |
| GP5a | 64 | 5a | Minor structural protein |
| M | 162 | 6 | Integral membrane protein, part of the GP5/M heterodimer |
| N | 111 | 7 | Nucleocapsid protein, forms dimers, partially localizes to the nucleus of infected cells, phosphoprotein, variable in length for PRRSV-1 and PRRSV-2 (125, 126, 129, 131 and 124, 125 amino acids, respectively) |
Lipids
Virion lipids are cell derived. Nucleocapsids form in the cytosol and bud through the endoplasmic reticular membrane.
Carbohydrates
Although the number of predicted N-linked glycosylation sites in GP2−GP5 of EAV, PRRSV and LDV strains differ, the large majority of these sites are highly conserved and are present in almost all of the natural isolates of these viruses. Conserved N-linked glycosylation sites in GP5 are essential for infectivity and antigenicity of EAV and PRRSV. The EAV GP5 carbohydrate is modified with N-acetyllactosamine, while the PRRSV GP5 is thought to contain complex sugars other than polylactosaminoglycans.
Genome organization and replication
Cell attachment and entry
The tropism of arteriviruses for macrophages and dendritic cells depends on the presence or absence of specific entry mediators. PRRSV virions initially attach to macrophages through interactions with heparan sulphate glycosaminoglycans that line the cell surface. Subsequently, sialic acids on the glycan trees of the ectodomains of the viral GP5/M heterodimer bind to macrophage-specific lectins, such as Siglec-1 (CD169, sialoadhesin), Siglec-10 and DC-SIGN (Xie et al., 2017, Huang et al., 2009, Van Breedam et al., 2010). PRRSV binding to these lectins trigger uptake of the virus-receptor complex into macrophages via clathrin-mediated endocytosis. Upon endosome acidification, fusion of the viral and endosome membranes occurs, followed by release of the viral nucleocapsid into the cytoplasm. CD163 is essential in this disassembly process (Van Gorp et al., 2010). GP2 and GP4 have been identified as binding partners for CD163 by co-precipitation experiments, indicating that these molecules may also function in genome release. CD163-null pigs are resistant to PRRSV; CD169-null pigs are still susceptible (Whitworth and Prather 2017). The latter may be explained by the existence of multiple binding receptors. The transmembrane form of equine CXCL16S acts as a virus entry receptor in EAV susceptible equine CD3+ T cells, and monocytes (Sarkar et al., 2016, Sarkar et al., 2016). Similar to CD163, CXCL16 belongs to the scavenger receptor superfamily of proteins which are highly diverse in structure.
Synthesis of virus RNA: replication and transcription
Virion attachment is followed by virion endocytosis, membrane fusion, and release of the genome into the cytoplasm where its 5′-ORF1a and ORF1b are translated into the polyproteins pp1a and pp1ab that are cleaved into the mature viral non-structural proteins (nsps). Some of these nsps induce double-membrane vesicles (DMVs) with which the large viral RNA replication/transcription complexes (RTC) associate and replicate/transcribe viral RNAs. Continuously transcribed, full-length negative-sense RNAs as well as a 3′-5′ coterminal, nested set of subgenomic negative-sense RNAs generated by discontinuous transcription, that subsequently function as templates for the structural gene subgenomic mRNAs, are synthesized from the genome (Pasternak et al., 2006). The synthesis of the subgenomic negative-sense strands, which involves the fusion of noncontiguous 3′- and 5′-sequences, occurs by a discontinuous transcription process. Transcription-regulating sequences (TRSs) in the genome RNA mediate attenuation (3′-TRSs) and reinitiation (5′-leader TRS) during the synthesis of individual subgenomic negative-sense strands. For SHFV, multiple adjacent 3′-TRS sequences mediate 5′-translocation of nascent negative-sense transcripts for the majority of the 3′-genes (Di et al., 2017). Subgenomic SHFV mRNAs encoding several additional small ORFs have been identified (Di et al., 2017) and heteroclite (abnormal) subgenomic RNAs have been detected in PRRSV infected cells (Yuan et al., 2000). Nucleocapsid dimers and a nascent genome RNA associate to spherical capsids in the cytoplasm which then bud into the lumen of the endoplasmic reticular membrane in regions with inserted viral envelope proteins. Virions are transported through the secretory pathway and released by endocytosis.
Nonstructural proteins: synthesis, processing and function
The arterivirus genome is polycistronic (Snijder et al., 2013). The genomes of the members of five of the arterivirus subfamilies encode 10 functional ORFs, 8 of which encode structural proteins. The genomes of members of the Simarterivirinae subfamily encode four extra ORFs (14 ORFs in total), due to a large duplication in the minor structural gene region. Viruses of the four subfamilies, Simarterivirinae, Variarterivirinae, Heroarterivirinae, and Crocarterivirinae, encode two other functional ORFs in the ORF1a nsp2 area, known as the nsp2 transframe (TF) ORF in the -2 reading frame (equivalent to +1 RF specified in Figure 2. Arteriviridae), and also a single-codon ORF in the -1 reading frame compared to ORF1a (located in the 0 RF). The EAV and WPDV genomes do not contain either of these additional ORFs. Arteriviruses may also use small ORFs recently described through high-resolution analysis of SHFV subgenomic mRNAs (Di et al., 2017). Three-quarters of the 5′-proximal length of the genome is occupied by two large ORFs (ORF1a and ORF1b), collectively referred to as the viral “replicase” and together, with the optional involvement of the nsp2 ORFs in the alternative RFs, encode all components of the poorly characterized membrane-bound replication-transcription complex (RTC) that mediates genome RNA replication and the production of subgenomic mRNAs (Fang and Snijder 2010) (Table 3. Arteriviridae and Figure 2. Arteriviridae). ORFs 1a and 1b are translated into polyproteins that range in size among the studied arteriviruses from 1727 to 2502 aa for pp1a and 3175 to 3959 for pp1ab, with the latter being a C-terminally extended version of the former. ORF1b translation depends additionally on a -1 ribosomal frameshift (RFS) occurring just before termination of ORF1a translation (Li et al., 2019). With the exception of EAV and uncertainty about wobbly possum disease virus (WPDV), translation of ORF1a is prematurely terminated by a -1 and -2 RFS by a fraction of the ribosomes to produce two pp1a truncated products in the nsp2 genome area, known as nsp2TF and nsp2N, respectively (neither is shown in Figure 2. Arteriviridae). The polyproteins pp1a and pp1ab are autoproteolytically processed by 3−5 cognate proteases at conserved sites into 13−17 single- and multi-domain nsps. ORF1a and ORF1ab polyprotein cleavage sites have been determined for EAV and with the exception of the nsp1 and 2 proteins have been deduced for other arteriviruses by homology (Li et al., 2015). Conserved arterivirus nsp domains include (from N-terminus to C-terminus): a zinc finger (ZF in nsp1, except for WPDV), nsp1 papain-like proteinases (PLPα and PLPβ, and also PLP1γ in SHFV and other viruses of the subfamily Simarterivirinae; PLPα is enzymatically inactive in EAV and the identity of these enzymes is currently tenative for WPDV; a papain-like proteinase that represents a unique subclasss of zinc-dependent OTU deubiquitinases (PLP2) present in nsp2 and if they are produced, in nsp2TF and nsp2N; a nidovirus conserved chymotrypsin-like serine proteinase (SP) in nsp4; a nidovirus RNA-polymerase associated nucleotidyl-transferase (NiRAN) and an RNA-directed RNA polymerase (RdRp) in nsp9; a nidovirus conserved zinc binding domain (ZBD) covalently bound through a linker domain to an NTPase/RNA helicase of superfamily 1 (HEL1) in nsp10; a nidovirus uridylate-specific endoribonuclease (NendoU) in nsp11; and an arterivirus-specific replicase subunit devoid of 2′ O-methyltransferase activity in nsp12 (AsD) that serves as a genetic marker of the family. NiRAN and ZBD are exclusively conserved in all nidoviruses and serve as genetic markers of the order, and NendoU is conserved in most vertebrate nidoviruses, but not found in other viruses. Various non-structural proteins contain arterivirus- and/or nidovirus-specific domains, as specified above, or signatures, including an SDD signature (instead of the canonical GDD) in the RNA-directed RNA polymerase (RdRp) (nsp9) of all arteriviruses except WPDV, which possesses GDD. The nsp1s of EAV and PRRSV-2 have been shown to be critical regulators of the levels of genomic and subgenomic mRNAs. The proteins nsp2 and nsp3, which have hydrophobic domains, have been implicated in the formation of the endoplasmic reticulum-derived DMVs and the viral RTC anchoring to DMVs. Arterivirus infections utilize multiple means of modulating the host innate immune response. PRRSV-2 nsp1α and nsp1β modulate the type I IFN response. The arterivirus PLP2 protein disrupts innate immune signaling by removing ubiquitin and ubiquitin-like moieties from host proteins. Three small proteins, nsp7α, nsp7β and nsp8, are believed to assist ORF1b-encoded enzymes of the RTC in yet to be defined roles. Many proteins may have other activities mediating virus-host interaction that are covered elsewhere in this chapter.
Table 3. Arteriviridae. Nonstructural proteins of arterivirusesa
| Protein | aab | Mode of expressionc | Putative Function |
nsp1αd
nsp1β | 180
203 | TI + nsp1 PLP1α
TI + PLP1β | Zinc finger (ZnF), proteinase (PLP1), replicase polyprotein processing, transcription, and virion biogenesis (dispensable for genome replication), innate immune response counteraction. Proteinase (PLP2), replicase polyprotein processing, innate immune response counteraction. |
nsp2
nsp2TF
nsp2N | 1196
1019
850 | TI + nsp1 PLP1β or also PLP1γ + nsp2 PLP2
TI + nsp1 PLP1β or also PLP1γ + RFS
TI + nsp1 PLP1β or also PLP1γ + RFS | Proteinase (PLP2) with cis and trans cleavage activity (Han et al., 2009) and deubiquitinating activity (DUB) (van Kasteren et al., 2013), integral membrane protein, formation of double membrane vesicles for viral RNA replication, PRRSV nsp2 contains a hypervariable region and variable numbers of a novel PxPxPR motif, phosphoprotein. Isoforms of nsp2 can be detected in PRRSV virions (Kappes et al., 2013). Truncated nsp2 with a unique C-terminus due to a −2 ribosomal frame shift that is enhanced by nsp1β and poly(rC) binding proteins. (not produced by EAV, uncertain for WPDV).
Truncated nsp2 with a unique C-terminus due to a −1 ribosomal frame shift enhanced by nsp1β and poly(rC) binding proteins. (not produced by EAV, uncertain for WPDV) |
| nsp3 | 230 | TI + nsp2 PLP2 + nsp4 SP | Integral membrane protein, formation of double membrane vesicles for viral RNA replication |
| nsp4e | 204 | TI + nsp4 SP | Main proteinase with chymotrypsin-like fold (SP) and extra C-terminal domain responsible for most cleavages of pp1a/pp1ab |
| nsp5 | 170 | TI + nsp4 SP | Integral membrane protein, replication complex (DMV) formation. Exogenous expression of nsp5 induces small diameter autophagosomes. |
| nsp6 | 16 | TI + nsp4 SP | Exogenous expression of nsp6 induces small diameter autophagosomes. |
| nsp7α, β | 259 | TI + nsp4 SP | Nsp7α is the most conserved region of nsp7, functions as a distinct replicase domain and has a novel fold (Manolaridis et al., 2011). Exogenous expression of nsp7 induces small diameter autophagosomes. |
| nsp8f | 45 | TI + nsp4 SP + TT | The most C-terminal ORF1a-encoded protein; overlaps with nsp9, function unknown |
| nsp9 f | 685 | TI + RFS + nsp4 SP | The N terminal region overlaps with nsp8; NiRAN, nidovirus RNA-polymerase associated nucleotidyltransferase (Lehmann et al., 2015) ; RdRp, RNA-directed RNA polymerase (Lehmann et al., 2016) |
| nsp10 | 441 | TI + RFS + nsp4 SP | RNA helicase/NTPase, flanked and assisted with the nidovirus conserved N-terminal zinc binding domain (Deng et al., 2014, Shi et al., 2020), role in subgenomic and genomic mRNA synthesis |
| nsp11 | 223 | TI + RFS + nsp4 SP | NendoU, nidovirus uridylate-specific endoribonuclease (Zhang et al., 2017) |
| nsp12 | 153 | TI + RFS + nsp4 SP + TT | Arterivirus-specific replicase subunit devoid of 2′ O-methyltransferase activity (Lehmann et al., 2015) |
a Based on the replicase processing scheme determined for EAV (van Dinten et al., 1996).
b aa = amino acids. Protein lengths shown are from PRRSV-1 AF046869.
c TI, translation initiation; RFS, ribosomal frame shift; TT, translation termination; PLP, papain-like cysteine proteinase; SP, serine (main) proteinase.
d The nsp1 region may include two or three papain-like proteinase (PLP) domains, named PLP1α, PLP1β, and PLP1γ, respectively. PLP1α is enzymatically inactive in EAV, while the EAV PLP1β may be orthologous to PLP1γ of other arteriviruses. The nsp1 region of SHFV and presumably other viruses of the subfamily Simarterivirinae is cleaved into three proteins nsp1α, nsp1β and nsp1γ (Vatter et al., 2014), while the nsp1 region of other arteriviruses except EAV is cleaved into nsp1α and nsp1β (Chen et al., 2010, den Boon et al., 1995). The nsp1α subunit of PRRSV contains two distinct zinc finger configurations, the second of which has been shown to be functional. Crystal structures have been determined for PRRSV-2 nsp1α (Sun et al., 2009)(Protein Data Bank ID 3IFU) and PRRSV-2 nsp1β (Protein Data Bank ID 3MTV).
e Crystal structures have been determined for EAV and PRRSV-2 nsp4 (Protein Data Bank IDs 1MBM and 3FAO, respectively).
f Due to a -1 RFS by a fraction of ribosomes during translation of the nsp8 locus, the pp1a-derived nsp8 and the pp1ab-derived nsp9 overlap.
Biology
Pathogenesis
All known arteriviruses infect a single type of vertebrate host in the domain Eucarya and are not vector-borne. Besides, macrophages and dendritic cells (see above), EAV can also infect stromal cells, CD3+ and CD8+ T-lymphocytes, and CD21+ B-lymphocytes and endothelial cells (Carossino et al., 2017, Vairo et al., 2013, Go et al., 2011). Arterivirus infections are cytocidal and cause either acute or persistent infections in their hosts. Most EAV infections are subclinical in nature, and may go undiagnosed. However, some virulent strains periodically cause significant outbreaks of disease, and can be associated with abortion, neonatal mortality, and the establishment of persistent infection in stallions. EAV establishes long-term persistent infection (LTPI) in the reproductive tract of stallions (Carossino et al., 2019, Carossino et al., 2018). The establishment of EAV LTPI correlates with the in vitro susceptibility of a subpopulation of CD3+ T lymphocytes to EAV infection, and, consequently, stallions with the CD3+ T lymphocyte susceptibility phenotype are at higher risk of becoming long-term persistently infected carriers compared to those that lack this phenotype (Go et al., 2011, Go et al., 2010). Two allelic variants of the CXCL16 gene that differ by four non-synonymous nucleotide substitutions in exon 1 show a very strong association with either the establishment of long-term persistence (CXCL16S) or early viral clearance in stallions (CXCL16R) (Sarkar et al., 2016, Go et al., 2011).
Pigs of all ages in immunologically naïve herds are susceptible to infection with PRRSV-1 and PRRSV-2 (Lunney et al., 2010). Clinical signs of porcine reproductive and respiratory syndrome (PRRS) are extremely variable and influenced by the strain of the virus, the immune status of the herd, and management practices. Low-virulence strains of PRRSV-1 and PRRSV-2 may result in widespread infection of pigs with minimal occurrence of disease, whereas highly virulent strains can cause severe clinical disease in susceptible herds. The infection of swine with PRRSV-1 and PRRSV-2 results in reproductive failure in sows, respiratory illness in growing swine, and is usually asymptomatic in boars. PRRSV-1 and PRRSV-2 strains can be extremely diverse, due in part to high levels of viral recombination that leads to variation in clinical symptoms from mild to severe. PRRSV-1 and PRRSV-2 can establish a persistent infection in the lymph nodes (e.g., inguinal, mandibular, and sternal) and tonsils of infected boars and sows, as well as in the reproductive tract of infected boars. Similar to EAV, PRRSV-1 and PRRSV-2 shedding in semen occurs in the absence of viremia and in the presence of neutralizing antibodies in serum. The presence of PRRSV-1 and PRRSV-2 in the semen of infected boars could be due to either local virus replication and shedding from reproductive tract tissues or more likely dissemination via infected monocytes and macrophages from other tissues.
LDV causes lifelong asymptomatic, persistent infections in mice that can only be recognized by elevated levels of plasma lactate dehydrogenase (LDH) (Plagemann et al., 1995). Persistent LDV infections in mice are maintained by replication of LDV in permissive macrophages that are continuously regenerated from apparently non-permissive precursor cells. Most virus stocks contain both neuropathogenic (LDV C and LDV v) and non-neuropathogenic (LDV P and LDV vx) variants (Cafruny et al., 2003). CNS disease resulting in paralysis was first observed in LDV C infected Fv-1 n/n mice (Martinez et al., 1980).
Before the advent of metagenomics, SHFV was thought to include all variants that infect monkeys. Recently many different simian arteriviruses classified as separate virus species of the subfamily Simarterivirinae have been identified (Bailey et al., 2016). Most of these viruses naturally infect a single species of African monkey, causing acute or persistent infections with no overt disease signs. However, SHFV, Pebjah virus (PBJV), and simian hemorrhagic encephalitis virus (SHEV) may induce fatal hemorrhagic disease in captive Asian macaques (Vatter et al., 2015, Wahl-Jensen et al., 2016, Lauck et al., 2015). The high fatality rate observed after laboratory infections in macaque monkeys may be due to the extreme sensitivity of their macrophages and dendritic cells to cytocidal infection by SHFV and the high levels of virus and proinflammatory cytokines produced by infected cells (Vatter and Brinton 2014).
Arteriviruses have recently been found in multiple species of rodents, shrews, and bank voles by sequence analyses but do not appear to be associated with disease (Wu et al., 2018, Kesaniemi et al., 2019). WPDV was found in brushtail possums in New Zealand and Australia. The virus was so named because infected animals have an uncoordinated “wobbly” gait (Gulyaeva et al., 2017). An arterivirus found in European hedgehogs was associated with a fatal encephalitis (Dastjerdi et al., 2021).
Antigenicity
EAV, PRRSV-1, PRRSV-2, LDV, and SHFV are antigenically distinct with no serological cross-reactivity except for some epitopes shared by the two genotypes of PRRSV (Oleksiewicz et al., 2002). Different strains of EAV, LDV, and PRRSV-1 and PRRSV-2 show significant antigenic variation. The very high degree of genotypic and phenotypic diversity in PRRSV-1 and PRRSV-2 strains from around the world has been a significant obstacle to developing an efficacious vaccine against porcine arteriviruses (Kimman et al., 2009, Kappes and Faaberg 2015). This variability supports the assignment of these viruses as two different species, Betaarterivirus suid 1 (including PRRSV-1) and Betaarterivirus suid 2 (including PRRSV-2).
Arterivirus infections elicit weak adaptive immune responses, and often result in persistent infections (Balasuriya and MacLachlan 2004, Carossino et al., 2017). Arterivirus infections typically elicit the production of high levels of antibody to the major structural proteins (GP5, M, and N), and multiple nsps (e.g., nsp1, nsp2, and nsp7 of PRRSV-2, and nsp2, nsp4, nsp5, and nsp12 of EAV) after natural and experimental infections (Go et al., 2011). The antibody response to the minor structural proteins of arteriviruses is poorly characterized. The major neutralization epitopes of EAV, PRRSV-1, PRRSV-2, SHFV, and LDV have been mapped to the amino-terminal ectodomain of the major envelope glycoprotein GP5 which contains four major neutralization sites (A, B, C, and D) (Balasuriya and MacLachlan 2004). Site D may interact with the three other sites to form conformational epitopes. Two major antigenic sites (Site A and Site B) are located in the amino-terminal ectodomain of the PRRSV-2 GP5, but only Site B can induce neutralizing antibodies in pigs. The immunodominant non-neutralizing epitopes in Site A may act as a decoy and delay the neutralizing antibody response against epitopes in Site B. GP5 and M heterodimerization is critical for the formation of conformational neutralization epitopes in the neutralization sites of EAV and PRRSV-2 GP5. Horses infected with EAV develop neutralizing antibodies by 1–2 weeks postinfection, while pigs infected with PRRSV-1 and PRRSV-2 do not develop neutralizing until 4–5 weeks. The minor envelope protein GP4 of the prototype European PRRSV-1 strain, Lelystad virus, carries a highly variable neutralization site (Costers et al., 2010). However, antibodies against this site do not cross react with the GP4 protein of the prototype North American PRRSV-2 strain VR2332. B cell epitopes have also been identified for a PRRSV-1 and PRRSV-2 nsp2 protein, but the importance of these epitopes in protective immunity against any PRRSV infection has not been determined.
LDV infections in mice are characterized by a lifelong viremia, despite the induction of antiviral innate and adaptative immune responses. Observed modifications to the immune microenvironment of infected mice, includes macrophage and natural killer cell activation, secretion of pro-inflammatory cytokines, modulation of T helper cell differentiation and polyclonal activation of B-lymphocytes. This modified immune microenvironment results in protection against some diseases such as allergies, graft-versus-host reaction, experimental autoimmune encephalitis and the growth of some tumors, but it exacerbates other pathologies, such as endotoxin shock and autoantibody-mediated autoimmune diseases (Coutelier 2014, Gaignage et al., 2017). Anti-LDV antibodies first appear 4–5 days post infection with peak titers at 3–4 weeks. Anti-GP5 antibodies that neutralize LDV do not appear in mice until 4 weeks post infection. Reduced binding of neutralizing antibodies to the GP5s of some strains of LDV and PRRSV-2 appears to be due to the presence of 2 to 4 predicted glycosylation sites in the GP5 N-terminal ectodomain (Plagemann et al., 1995, Plagemann 2001).
Loss of these GP5 glycosylation sites alters LDV cellular tropism, leading to infection of spinal cord anterior horn neurons and subsequent paralysis of the infected mice. There is also evidence of antibody-dependent enhancement by anti-LDV and PRRSV-2 antibodies that may play a major role in the pathogenesis and persistence of these viruses.
SHFV induces neutralizing antibodies in patas monkeys 7 days after an experimental infection. However, only low levels of anti-SHFV antibodies were found in many persistently infected patas monkeys. Neutralizing antibodies against one “strain” of SHFV did not completely neutralize other strains, indicating variation in the neutralization determinants of what were likely different species of simian arteriviviruses (Gravell et al., 1986). Based on its hydrophobicity, membrane topology and putative N-linked glycosylation sites, the SHFV GP5 is predicted to contain the major neutralization determinants. A decrease in the PBMC CD4+, CD8+, CD14+ and CD20+ cell populations was detected between 2 and 6 days post infection of Japanese macaques with SHFV consistent with the existence of an immunosuppressive state. The number of CD4+ and CD8+ cells increasing thereafter. A transient increase in the CD14+ and CD20+ populations was also observed (Vatter et al., 2015).
Anti-viral CD8+ (cytotoxic lymphocytes [CTLs]) and CD4+ T cell responses have been detected in arterivirus-infected animals. T-cell epitopes were identified in the GP4, GP5, M, N (PRRSV-1 and -2), and nsp9 and nsp10 proteins of PRRSV-2 (Costers et al., 2010, Bautista et al., 1999, Diaz et al., 2009, Parida et al., 2012). PRRSV-specific T-cell proliferative responses (both CD4+ and CD8+) in pigs infected with PRRSV-1 or PRRSV-2 occur between 4 and 12 weeks after infection (Bautista and Molitor 1997, Albina et al., 1994). Activation of T lymphocytes by LDV infection occurs by one day postinfection, triggered by the large amounts of IFNα produced by productively-infected macrophages, and followed by transient suppression of T cell responses (cytotoxic and helper) that is maximal at about three days postinfection. In LDV infected mice, the CTLs generated are directed at epitopes that are located in the M, GP2b, and GP5 proteins. However, LDV persists in the blood of infected mice despite the presence of virus specific cell-mediated immune responses.
Subfamily, genus and species demarcation criteria
The taxonomy of the family Arteriviridae is based on DEmARC analyses (Lauber and Gorbalenya 2012) of the genetic divergence in the multiple sequence alignment (MSA) of five concatenated protein domains (3CLpro, NiRAN, RdRp, ZBD and HEL1). It has been advanced several times since the 9th report by the Arteriviridae Study Group in 2012 (Faaberg et al., 2012, Kuhn et al., 2016). The most recent dataset included 1155 genome sequences, with WPDV being the most divergent arterivirus. Pairwise patristic distances (PPD), calculated for FastTree 2.1.4 SSE3 maximum-likelihood (ML) phylogeny of arteriviruses, were analysed by DEmARC to identify candidate distance thresholds for taxonomy ranks. We then selected either a range or a particular value of PPD which supported already established taxa, starting with the species rank, and used these distances as the family-wide demarcation criterion for taxa at each of four ranks: subfamily, genus, subgenus, and species (Table 4.Arteriviridae). Technically, they correspond to thresholds of local minima in the clustering cost (CC) distribution, commonly with the CC=0 and largest ranges of PPD that reflect discontinuity in the underlying PPD distribution. Subfamily and subgenus ranks were introduced in 2017 to utilize as many available local CC=0 minima as possible in the taxonomy; however, the number of deep CC minima (potential thresholds) still exceeds the number of ranks in the taxonomy. The generous choice of thresholds may reflect the current limited sampling of the natural arterivirus diversity, which must be addressed for the ranks and the taxa of the arterivirus taxonomy when finalized. Since the demarcation criteria at all ranks are set family-wide, a taxon at any rank may include a single virus (genome sequence), which is indeed the case for many taxa.
Table 4.Arteriviridae. Demarcation thresholds for four ranks of the family Arteriviridae
| Rank | Number of taxa | PPD range1 | PUD (%) range2 |
| subfamily | 6 | 0.952-1.502 | 0.397-0.497 |
| genus | 13 | 0.578-0.637 | 0.290-0.310 |
| subgenus | 11 | 0.281-0.361 | 0.165-0.203 |
| species | 23 | 0.174-0.196 | 0.107-0.120 |
1Demarcation threshold depicted as a range of PPD values for which the number of clusters (taxa) remained constant and CC=0. PPD values account for repeated replacements of amino acid residues at sites and may exceed one.
2Demarcation threshold depicted as a range of pairwise uncorrected distances (PUD) that may vary from 0 to 100% of different residues in a pair of compared proteins; it was deduced from PPD values.
Derivation of names
Family
Arteriviridae: derived from name of a disease in horses caused by equine arteritis virus.
Subfamily (names are based on either host species or virus characteristics of members, with the common ending “arterivirinae”)
Crocarterivirinae: from Crocidura olivieri guineensis, the binomial name of African giant Olivier’s shrews from which first virus member in this subfamily was isolated
Equarterivirinae: from equine arteriviruses
Heroarterivirinae:- from HeroRATS, a name for the giant Gambian pouched rat, referring to their being trained as buried bomb sniffers, this animal being the host from which the first virus member in the subfamily was isolated
Simarterivirinae: from simian arteriviruses
Variarterivirinae: from the subfamily including viruses infecting various hosts (pigs, rats and mice)
Zealarterivirinae: from New Zealand, the location where the first virus member in this subfamily was isolated
Genus - Names of all genera taxa include ‘arterivirus’ preceded by a prefix corresponding to a Greek alphabet letter as written in English. Greek letters were assigned in alphabetical order according to the relative timing of member virus discovery.
Subgenus - Names of all subgenera are formed from a unique part followed by the common ending ‘artevirus’, which is a shortened version of arterivirus. The unique part ends with the first letter of the corresponding genus name.
Species – A non-Latinised binomial nomenclature, which includes the name of the respective genus, was adopted for most species. Names of a few species may also include a digit as a third component. See details in the relevant Genus/Genera sections
Relationships within the family
Viruses belonging to all arterivirus taxa occupy monophyletic clusters in a rooted ML tree produced using the amino acid multiple sequence alignment of five conserved domains (3CLpro, NiRAN, RdRp, ZBD and HEL1). Support for the clusters varies (Figure 3.Arteriviridae), and a few relatively poorly supported taxa were sensitive to an increase of virus sampling over time, resulting in changes in virus assignments to species and subgenera. Divergent arteriviruses have recently been identified in Asian rodents, African pouched rats, African giant shrews, European bank voles and New Zealand possums and it is expected that additional arteriviruses will be found in the future.
 |
| Figure 3.Arteriviridae. Phylogenetic tree of arteriviruses and the Arteriviridae taxonomy. The corresponding sequence alignment are available to download from the Resources page. |
A phylogenetic tree for 23 arteriviruses representing all species in the family was derived from a tree of representative nidoviruses and visualized using R package “ape” (Schliep 2011); non-arteriviruses served an outgroup to root the tree. The nidovirus tree was reconstructed by IQ-Tree 1.5.5, based on multiple sequence alignments of five protein domains (3CLpro, NiRAN, RdRp, ZBD and HEL1) with the evolutionary model for each domain selected independently. Branch support was estimated using Shimodaira-Hasegawa-like approximate likelihood ratio test (SH-aLRT) with 1000 replicates (Nguyen et al., 2015). Taxa names at the rank of species, subgenus, genus and subfamily are indicated.
Relationships with other taxa
Viruses in the family Arteriviridae are genetically most similar to those in other families of the order Nidovirales (Gorbalenya et al., 2006). These include the relatively well-characterized families Coronaviridae, and Tobaniviridae, members of which infect vertebrates, and the families Roniviridae and Mesoniviridae, members of which infect invertebrates, as well as nine other families of animal viruses largely known only from their genome sequence. All these viruses share important common features at the level of genome organization, genome expression strategy, phylogeny and internal organization of their large replicase gene. Conservation of the five replicative protein domains, 3CLpro, NiRAN, RdRp, ZBD and HEL1 enabled a common framework for taxonomies of the Arteriviridae and other nidovirus families. Despite these overall similarities, arterivirus genomes are substantially smaller than those of members of the families Coronaviridae, and Tobaniviridae, and the size, structure and composition of their virions do not resemble those of other characterized members of the order Nidovirales. Consequently, arteriviruses were described as small-genome nidoviruses while the members of the two other characterized families in the order were recognized as large-genome nidoviruses. Over the last ten years, this gap in genome size between the two groups has been gradually filled by newly identified nidoviruses, including those in the Tobaniviridae and those infecting invertebrates. Also, several recently described vertebrate nidoviruses known only from their genome sequence, were found to have relatively small genomes. Some of these viruses form three families that like the Arteriviridae belong to the suborder Arnidovirinae, while other viruses form two families of the suborder Nanidovirinae that is the sister taxa to viruses of the suborder Arnidovirinae. These viruses are basal to members of the Arteriviridae in the phylogenetic tree of the Nidovirales. Some of these viruses have names that include the stem “arteri”, since they were named before they were formally classified into separate families. Also, arteriviruses share similarities with viruses external to the order Nidovirales (Shi et al., 2018). These relationships were described in the 9th ICTV Report and are partly captured in the higher taxonomic ranks that include this order.

