Subfamily: Orthoretrovirinae
Genus: Deltaretrovirus
Distinguishing features
Viruses in this genus are most similar to viruses in the genus Gammaretrovirus, but can be distinguished based on RT-based phylogenetic clustering, by the presence of the regulatory genes tax and rex, and in some viruses, the unusual presence of an hbz gene on the antisense strand.
Virion
Morphology
Virion assembly and morphology is similar to gammaretroviruses.
Physicochemical and physical properties
See discussion under family description.
Nucleic acid
See discussion under family description.
Proteins
Approximate protein sizes are: matrix protein (MA) 19 kDa; capsid protein (CA) 24 kDa; nucleocapsid protein (NC) 12–15 kDa; protease (PR) 14 kDa; reverse transcriptase (RT), integrase (IN), surface envelope protein (SU) 60 kDa; and transmembrane envelope protein (TM) 21 kDa.
Genome organization and replication
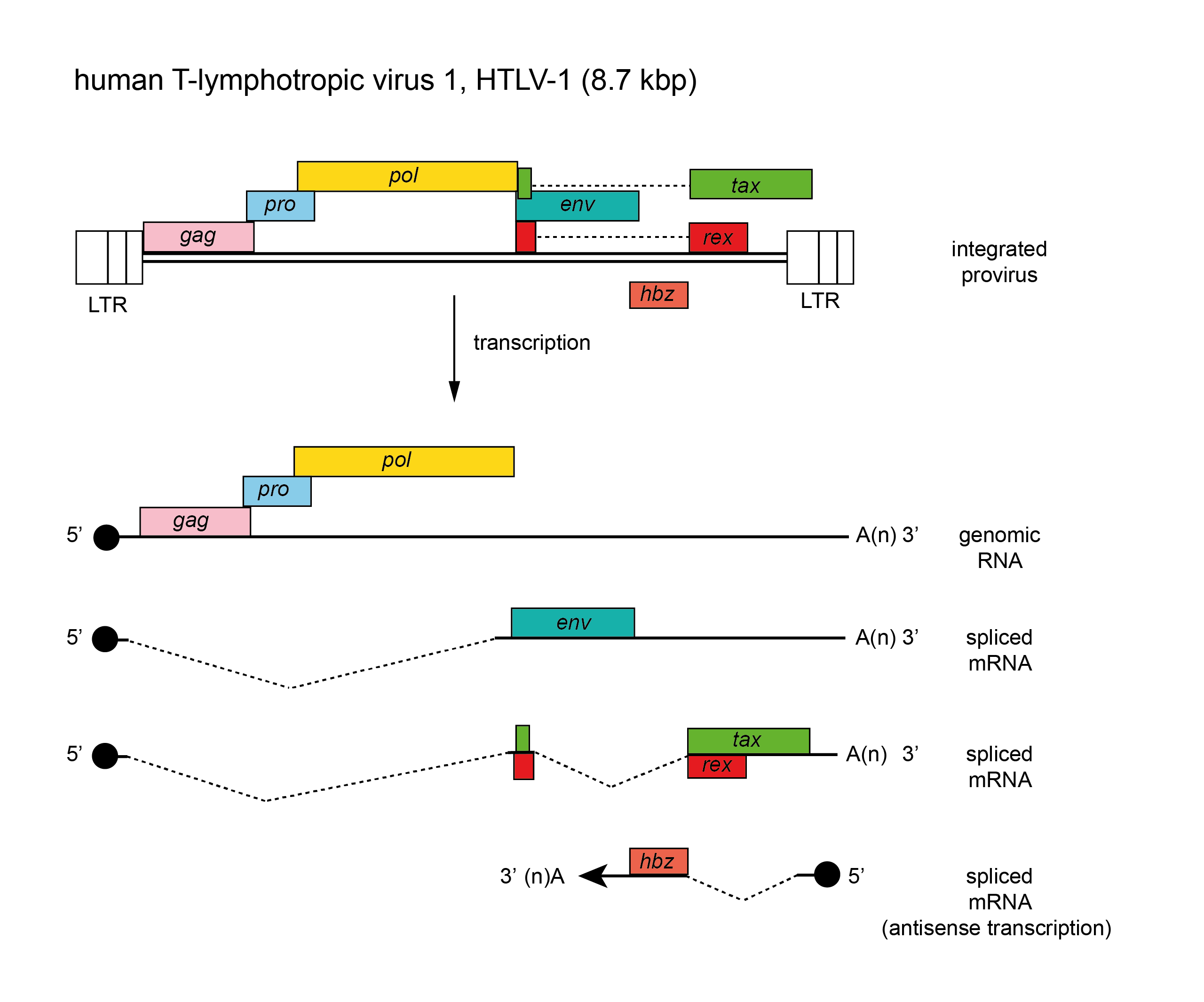
The genome is about 8.3 kb (one monomer) (Figure 1. Deltaretrovirus). In addition to gag, pro, pol and env, deltaretroviruses also encode two unique non-structural genes, tax and rex, which encode proteins involved in regulation of synthesis and processing of virus RNA. There is also an unusual gene encoded on the antisense strand, known as hbz. The tRNA primer is tRNAPro. The long terminal repeat (LTR) is about 550–750 nt, of which the U3 region is 200–300 nt, R is 135–235 nt and the U5 region is 100–200 nt.
 |
|
Figure 1. Deltaretrovirus. Deltaretrovirus genome organization. The 8.7 kbp human T-lymphotropic virus 1 (HTLV-1) provirus genome is shown, with LTRs, protein-coding regions (gag, pro, pol, env, tax and rex, and on the antisense strand, hbz) and transcripts (solid line arrows with protein names added) marked. The pro and pol genes are expressed as a result of ribosomal frameshifts. |
Biology
Exogenous viruses (horizontal transmission) in this genus are found in only a few groups of mammals (primates, cows) (Barez et al., 2015, Fujii and Matsuoka 2013). Related ERV sequences are extremely rare, but have been reported in the genomes of cetaceans and bats (Hron et al., 2019). Virus infections are associated with B or T cell leukemias or lymphomas as well as neurological disease (tropical spastic paraparesis or HTLV-associated myopathy) and exhibit a long latency (Fujii and Matsuoka 2013, Gessain and Cassar 2012). No oncogene-containing members of this genus have been identified.
Species demarcation criteria
Primate T-lymphotropic virus species are distinguished primarily on the basis of sequence differences, with viruses in each species belonging to several subtypes. All viruses in the genus have a similar coding strategy, but only human T-lymphotropic virus 1 (HTLV-1) has been associated with human disease. In addition to the canonical gag, pro, pol and env genes, viruses also encode the regulatory genes tax and rex. Phylogenetic trees containing HTLV-1 and simian T-lymphotropic virus 1 (STLV-1, Primate T-lymphotropic virus 3) do not recapitulate host species phylogeny; rather, viruses interleave and cluster according to geographic origin, suggesting that different HTLV-1 subtypes probably originated from separate interspecies transmissions from simians to humans.
Related, unclassified viruses
|
Virus name |
Accession number |
Abbreviation |
|
human T-lymphotropic virus 4 |
HTLV-4 |
|
|
simian T-lymphotropic virus 5 |
STLV-5 |
Virus names and virus abbreviations are not official ICTV designations.

