Family: Kolmioviridae
Genus: Deltavirus
Distinguishing features
Hepatitis D viruses 1–8 (HDV1–8) are the only kolmiovirids known to cause disease, and all of them infect humans (Dandri et al., 2022).
Virion
Morphology
Deltavirions are spherical (36–43 nm in diameter) with a lipid envelope containing peplomers obtained from hepatitis B virus (HBV, family Hepadnavridae) (Figure 1 Deltavirus). In vitro evidence suggests that HDV may utilize also other unrelated helper viruses in virion formation. Virions contain a ribonucleoprotein (RNP) complex consisting of about 70 copies of small delta antigen (S-DAg) and large delta antigen (L-DAg), which are de facto nucleoproteins that closely associate with deltavirus genomic and antigenomic RNA (Gudima et al., 2007).
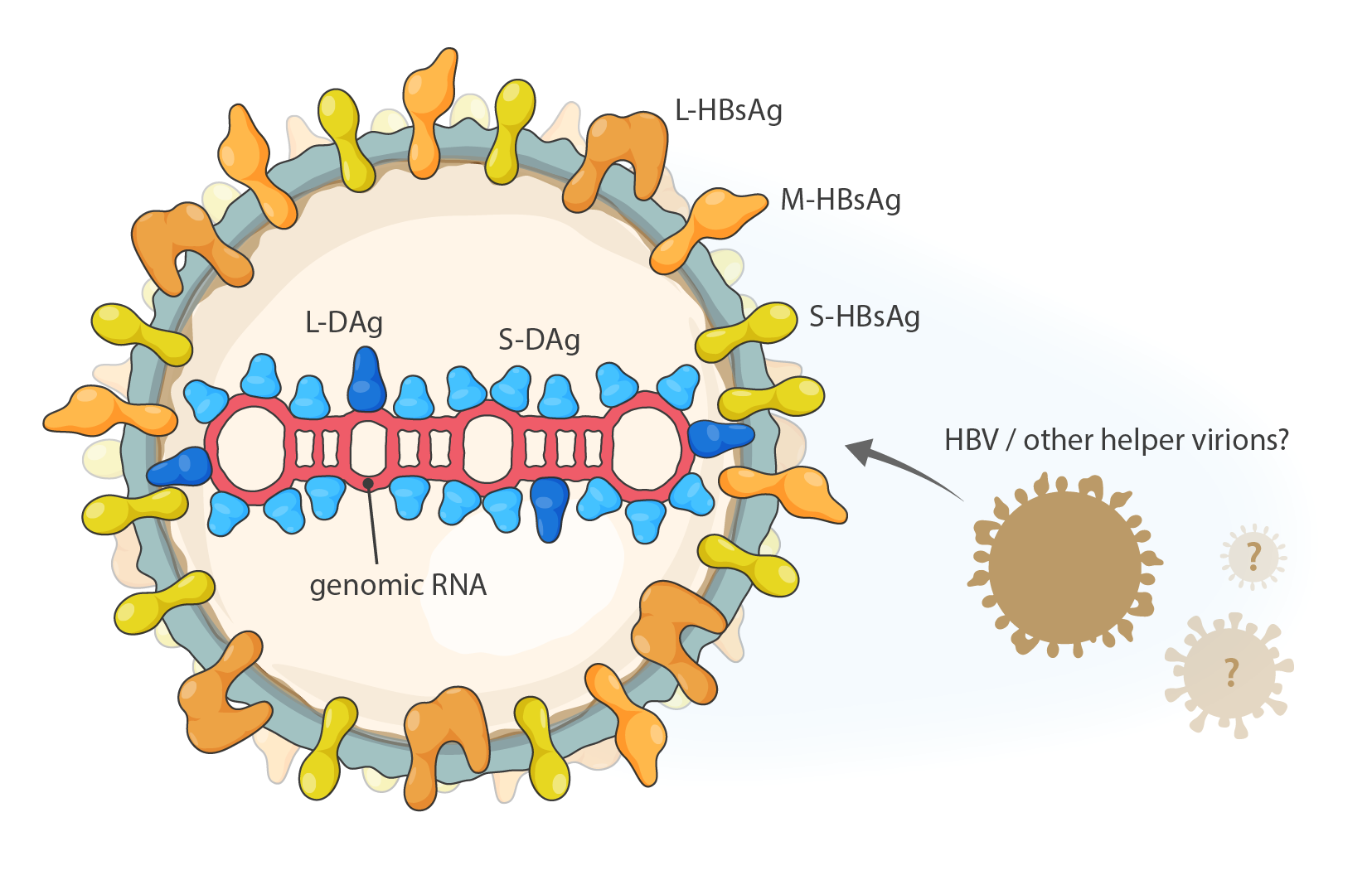 |
| Figure 1 Deltavirus. Schematic representation of the particles produced by the kolmiovirid hepatitis D virus 1. L-DAg, S-DAg: large and small delta antigen; L-HBsAg, M-HBsAg, S-HBsAg: large, middle and small hepatitis B virus surface antigen; HBV: hepatitis B virus. |
Physicochemical and physical properties
Deltavirions decorated by HBV surface structures have a buoyant density of approximately 1.24 g cm−3 in CsCl (Sureau et al., 1992).
Nucleic acid
Deltaviruses have non-segmented, negative-sense, covalently-closed circular RNA (cccRNA) genomes of about 1.7 kb with about 60% GC content (58.9% for AF104263). Genomes fold into unbranched, rod-like structures due to extensive (approximately 70%) intramolecular base pairing (Hoyer et al., 1983, Chen et al., 1986, Denniston et al., 1986, Kos et al., 1986, Wang et al., 1986, Kuo et al., 1988a). Genomes and antigenomes contain ribozymes (Kuo et al., 1988b).
Proteins
The deltavirus genome has an open reading frame encoding delta antigen (DAg) in the antigenomic orientation. DAg acts as the nucleoprotein of deltaviruses, and it comes in two isoforms, small DAg (S-DAg; previously also referred to as p24) of 194–195 amino-acid residues and large DAg (L-DAg; previously also referred to as p27) of 213–214 amino-acid residues (Kuo et al., 1989, Polson et al., 1996, Wille et al., 2018, Chang et al., 2019, Hetzel et al., 2019, Paraskevopoulou et al., 2020). The L-DAg is identical to the S-DAg but usually contains 19 (20 in case of HDV3) additional amino acid residues. Host-cell adenosine deaminase RNA specific (ADAR) converts the adenosine of the amber (UAG) stop codon in the antigenomic RNA to an inosine (UIG), resulting in an ACC in the edited genomic RNA, and an UGG codon in the L-DAg mRNA, with the stop codon (UAG) replaced by a tryptophan (UGG) codon, thereby extending the ORF (Kuo et al., 1989, Polson et al., 1996, Wille et al., 2018, Chang et al., 2019, Hetzel et al., 2019, Paraskevopoulou et al., 2020).
S-DAg and L-DAg are multifunctional and possess domains that mediate oligomerization via a coiled-coil structure, host cell nuclear translocation via a bipartite signal sequence, and RNA-binding via two arginyl-rich motifs. L-DAg has a domain that includes a prenylation site required for virion packaging. Both proteins are post-translationally acetylated and phosphorylated (Chang et al., 1988, Glenn et al., 1992, Xia et al., 1992, Moraleda et al., 2000).
Additional structural proteins in deltavirions are provided by helper virus and incorporated into the deltavirion envelope during budding.
Carbohydrates
Carbohydrates on the deltavirus particle have not been characterized. Since helper viruses provide deltavirion envelope and envelope proteins, deltavirion carbohydrates are likely to be similar to those found on helper virions.
Genome organization and replication
Deltavirion cell entry is mediated by its envelope proteins, which are obtained from helper viruses. Consequently, deltavirions bind and enter the same types of cells as the helper virus. For instance, the primary helper virus of HDV1–8, hepatitis B virus (HBV), enters human hepatocytes basolaterally via engagement of its S and preS1 surface protein domains with solute carrier family 10 member 1 (SLC10A1; previously referred to as Na+/taurocholate co-transporting polypeptide, NTCP) (Yan et al., 2012). Thus, in HDV1–8/HBV co-infections, HDV1–8 engage SLC10A1 as well (Yan et al., 2012).
Fusion of the deltavirion membrane with host-cell membranes releases the RNP into the cytosol, from where it migrates to the cell nucleus. There, deltavirus RNA-directed RNA (genome and antigenome) synthesis is mediated by host-cell DNA-directed RNA polymerase II (Taylor 1990, Taylor 2015) through a double rolling circle mechanism; genome- and antigenome-encoded ribozymes catalyze autocatalytic cleavage of concatenated progeny genomes and ligation/recyclization in the nucleus (Kuo et al., 1988b, Sharmeen et al., 1988, Branch et al., 1989, Wu et al., 1989, Perrotta and Been 1991, Ferré-D'Amaré et al., 1998). S-DAg functions as a transactivator of deltavirus RNA replication (Kuo et al., 1989), whereas L-DAg is essential for packaging and can in some situations inhibit replication (Sato et al., 2004, Taylor 2015). Full-length or truncated deltavirus RNA molecules are incorporated into virions as long as they are capable of folding into rod-like structures and binding to S- and L-DAg. Antigenomes are not incorporated into particles (Chen et al., 1986).
Biology
Deltaviruses infect humans and are primarily hepatotropic due to HBV’s cellular tropism. HDV1 circulates predominantly in China, Europe, and the USA; HDV2 and HDV4 in Japan and Taiwan; HDV3 in South America, and HDV5–8 in Africa. Natural recombinants of HDV1 and HDV2 have been described (Wu et al., 1999). Unclassified potential deltaviruses have been discovered in fat-tailed dunnarts (dasyurid Sminthopsis crassicaudata (Gould, 1844)) and Tasmanian devils (dasyurid Sarcophilus harrisii (Boitard, 1841)) sampled in Australia (Harvey et al., 2023).
Natural HDV1–8 infections primarily occur in humans infected with HBV. However, HDV1 can be transmitted to common chimpanzees (hominid Pan troglodytes (Blumenbach, 1775)) if accompanied by HBV and to woodchucks (sciurid Marmota monax (Linnaeus, 1758)) if woodchuck hepatitis virus (WHV; Hepadnaviridae: Orthohepadnavirus) is provided as the helper virus. HDV RNA and DAg were also reported to be present in minor salivary gland acinar, ductal, myoepithelial, and adipose cells in patients with Sjögren's disease in the alleged absence of HBV coinfection (Weller et al., 2016, Hesterman et al., 2023). This finding may sustain the suggestion that viruses other than HBV serve as helper viruses for deltavirus egress. Indeed, orthoflaviviruses, hepaciviruses, and vesiculoviruses can be functional helper viruses under experimental conditions (Perez-Vargas et al., 2019).
Deltavirus transmission mimics that of HBV, i.e., sexually or parenterally (e.g., needle sharing by intravenous drug users), whereas mother-to-child transmission during birth is uncommon for deltaviruses. Transmission of deltaviruses to individuals with chronic HBV infection (superinfection) typically leads to deltavirus persistence, whereas co-transmission of deltaviruses together with HBV to a naïve host typically leads to transient infection of both deltaviruses and HBV. The number of HDV co-infected individuals varies greatly among HBV carriers (Stockdale et al., 2020). The clinical presentation of acute and chronic deltavirus/HBV coinfection, designated as hepatitis D, is variable and covers a similar spectrum as that of isolated HBV infection (Sureau and Negro 2016): acute hepatitis, chronic active hepatitis, cirrhosis, fulminant acute hepatitis, and hepatocellular carcinoma. However, the frequency of severe sequelae and their rates of progression are significantly higher in chronic deltavirus/HBV coinfection than in isolated chronic HBV infection.
Antigenicity
The presence of antibodies to DAg is indicative of current or past infections (Rizzetto 2015).
Species demarcation criteria
Members of the same species have a minimum of 80% nt genome and 70% S-DAg aa identity and form a monophyletic cluster in phylogenetic analyses (Figure 3 Kolmioviridae).
Related, unclassified viruses
| Virus name | Accession number | Virus abbreviation | Reference |
| fat-tailed dunnartdeltavirus | (Harvey et al., 2023) | ||
| Tasmanian devil deltavirus | (Harvey et al., 2023) |
Virus names and virus abbreviations are not official ICTV designations.

