Family: Flaviviridae
Genus: Orthoflavivirus
Distinguishing features
The 5′-end of the genome possesses a type I cap (m7GpppAmp) not seen in viruses of the other genera. Most orthoflaviviruses are transmitted to vertebrate hosts by arthropod vectors, mosquitoes or ticks, in which they replicate actively. Some orthoflaviviruses transmit between rodents or bats without known arthropod vectors.
A Table with current (MSL38) and previous (MSL37) species names is available at Species names: Flaviviridae.
Virion
Morphology
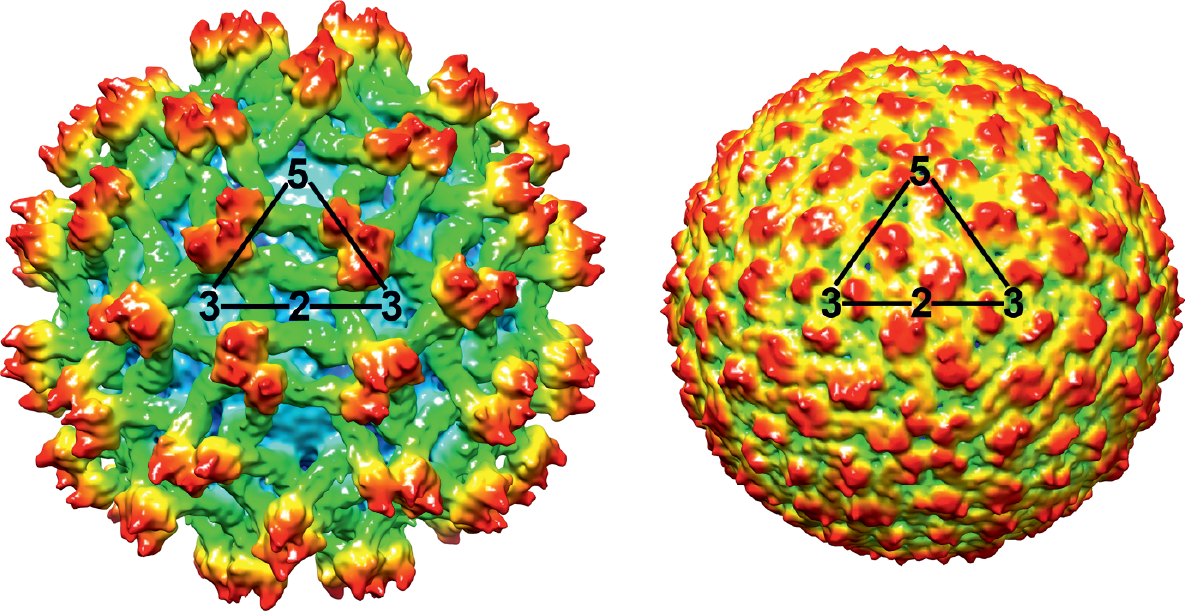
Virions are 50 nm in diameter and spherical in shape (Figure 1.Orthoflavivirus). Two virus forms can be distinguished. Mature virions contain two virus encoded membrane-associated proteins, E and M. Intracellular immature virions contain the precursor prM, which is proteolytically cleaved into M during maturation (Stadler et al., 1997). In certain instances, partially mature/immature forms are also released from infected cells. The virion structures of dengue virus (DENV) and West Nile virus (WNV) have been determined by X-ray crystallography (Kuhn et al., 2002, Mukhopadhyay et al., 2003). The envelope protein, E, is a dimeric, rod-shaped molecule that is oriented parallel to the membrane and does not form spike-like projections in its neutral pH conformation (Yu et al., 2008). Image reconstructions from cryo-electron micrographs (Figure 1.Orthoflavivirus) have shown that the virion envelope has icosahedral symmetry, in which E protein dimers are organized in a herringbone-like arrangement.
|
|
|
Figure 1.Orthoflavivirus. Three-dimensional cryo-electron microscopic reconstructions of immature (left) and mature (right) particles of an isolate of dengue virus (courtesy of M. Rossmann). Shown is a surface rendering of immature dengue virus at 12.5Å resolution (left) and mature dengue virus at 10Å resolution (right). The viruses are depicted to scale, but not coloured to scale. Triangles outline one icosahedral unit. |
Physicochemical and physical properties
Virion Mr has not been precisely determined. Mature virions sediment at about 200S and have a buoyant density of about 1.19 g cm−3 in sucrose (Kokorev et al., 1976). Viruses are stable at slightly alkaline pH 8.0 but are readily inactivated by exposure to acidic pH, temperatures above 40 °C, organic solvents, detergents, ultraviolet light and gamma-irradiation.
Nucleic acid
The virion RNA of orthoflaviviruses is a positive-sense infectious ssRNA of 9.2–11.0 kb. The 5′-end of the genome possesses a type I cap (m-7GpppAmp) where the A is followed by a highly conserved G nucleotide. The 3′-ends lack a terminal poly(A) tract and terminate with the conserved dinucleotide CU.
Proteins
Virions contain three structural proteins: capsid (C, 11 kDa), the major envelope protein (E, 50 kDa), , and either prM (26 kDa), in immature virions, or M (8 kDa), in mature virions. The E protein is the viral haemagglutinin, which mediates both receptor binding and acid pH-dependent fusion activity after uptake by receptor-mediated endocytosis. Seven nonstructural proteins are synthesized in infected cells: NS1 (46 kDa), NS2A (22 kDa), NS2B (14 kDa), NS3 (70 kDa), NS4A (16 kDa), NS4B (27 kDa) and NS5 (103 kDa). Some members of the genus harbour sequences that appear to induce a proportion of translating ribosomes to shift -1 nt and continue translating in the new reading frame to produce a 'transframe' fusion protein (Firth and Atkins 2009). When functionally utilized, this is referred to as programmed-1 ribosomal frameshifting (-1 PRF). NS1 has multiple forms and roles, with a cell-associated form functioning in viral RNA replication and a secreted form that regulates complement activation. One such form, a NS1′ protein, is the product of a −1 ribosomal frameshift and plays a role in viral neuroinvasiveness (Melian et al., 2010). The N-terminal one-third of NS1 forms the viral serine protease complex together with NS2B that is involved in processing the polyprotein. The C-terminal portion of NS3 contains an RNA helicase domain involved in RNA replication, as well as an RNA triphosphatase activity that is probably involved in formation of the 5′-terminal cap structure of the viral RNA. NS5 is the largest and most highly conserved protein that acts as the viral RdRP and also possesses methyltransferase activity involved in the modification of the viral cap structure.
Lipids
Virions contain about 17% lipid by weight; lipids are derived from host cell membranes.
Carbohydrates
Virions contain about 9% carbohydrate by weight (glycolipids, glycoproteins); their composition and structure are dependent on the host cell (vertebrate or arthropod). N-glycosylation sites are present in the proteins prM (1 to 3 sites), E (0 to 2 sites) and NS1 (1 to 3 sites).
Genome organization and replication
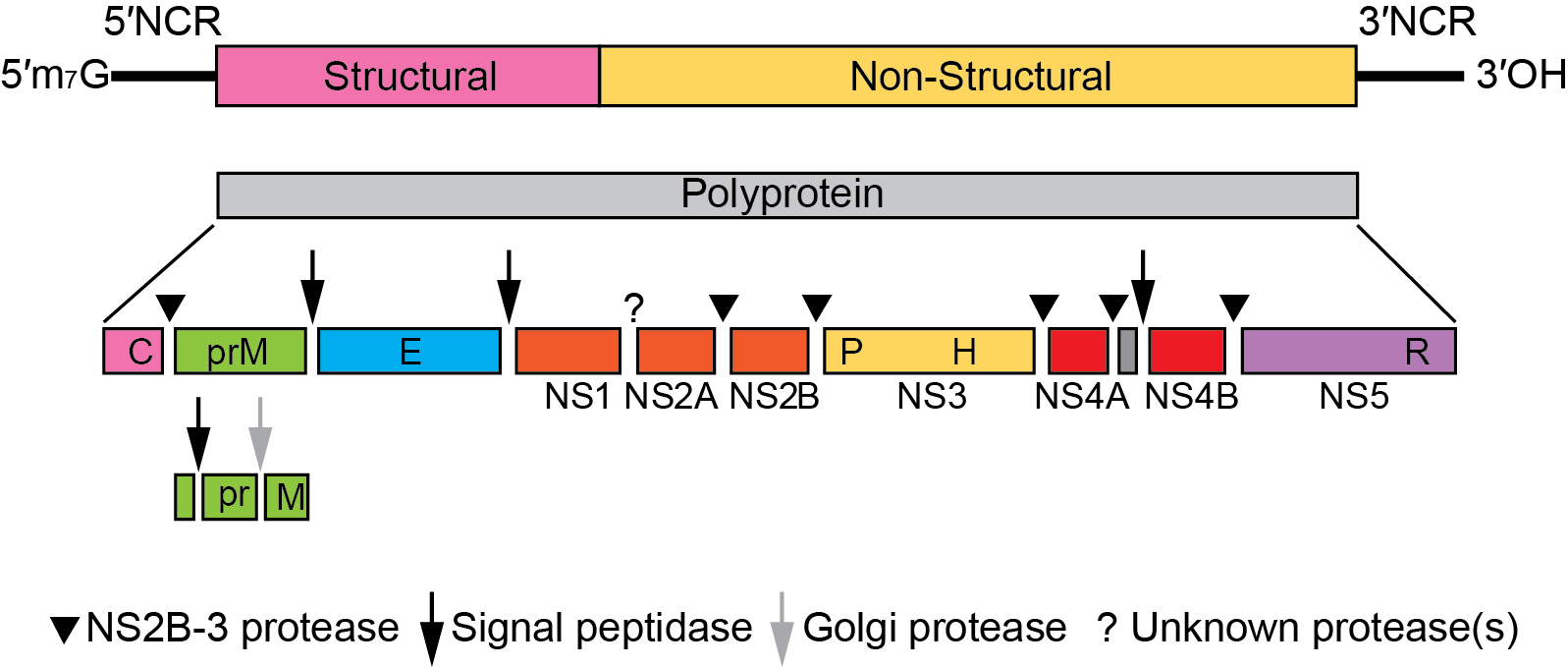
The genomic RNA represents the only viral messenger RNA in infected cells. It consists of a single long ORF of more than 10,000 nt that codes for all structural and nonstructural proteins and is flanked by NCRs at the 5′- and 3′-terminal ends (Figure 2.Orthoflavivirus).
|
|
|
Figure 2.Orthoflavivirus. Orthoflavivirus genome organization (not to scale) and polyprotein processing. The virion RNA is about 11 kb. At the top is the viral genome with the structural and nonstructural protein coding regions and the 5′- and 3′-NCRs. Boxes below the genome indicate viral proteins generated by the proteolytic processing cascade. P, H, and R symbols indicate the localization of the NS3 protease, the NS3 RNA helicase, and the NS5 RdRP domains, respectively. |
Both the 5′-NCR and the 3′-NCR contain RNA sequence motifs that are involved in viral RNA translation, replication and possibly packaging. Although RNA secondary structure and function of some elements are conserved, sequence composition, length and exact localization can vary considerably between different members of the genus, in particular between tick-borne and mosquito-borne orthoflaviviruses. In some cases, the 3′-NCR of tick-borne encephalitis virus, for example, contains an internal poly(A) tract. Viral infection induces dramatic rearrangements of cellular membrane structures within the perinuclear endoplasmic reticulum (ER) and causes the formation of ER-derived vesicular packets that most likely represent the sites of viral replication. After translation of the incoming genomic RNA, RNA replication begins with synthesis of complementary negative-strands, which are then used as templates to produce additional genome-length positive-stranded molecules. These are synthesized by a semi-conservative mechanism involving replicative intermediates (containing double-stranded regions as well as nascent single-stranded molecules) and replicative forms (duplex RNA molecules). Translation usually starts at the first AUG of the ORF, but may also occur at a second in-frame AUG located 12 to 14 codons downstream in mosquito-borne orthoflaviviruses. The polyprotein is processed by cellular proteases and the viral NS2B-NS3 serine protease to give rise to the mature structural and nonstructural proteins. Protein topology with respect to the ER and cytoplasm is determined by internal signal and stop-transfer sequences. Virus particles can first be observed in the rough endoplasmic reticulum, which is believed to be the site of virus assembly. These immature virions are then transported through the membrane systems of the host secretory pathway to the cell surface where exocytosis occurs. Shortly before virion release, the prM protein is cleaved by furin or a furin-like cellular protease to generate mature virions. Infected cells also release a non-infectious subviral particle that has a lower sedimentation coefficient than whole virus (70S rather than 200S) and exhibits haemagglutination activity.
Biology
Host range
Orthoflaviviruses can infect a variety of vertebrate species and in many cases arthropods. Some viruses have a limited vertebrate host range (e.g., only primates), while others can infect and replicate in a wide variety of species (mammals, birds, etc.). The usual route of infection for arthropods is when they feed on a viraemic vertebrate host, but non-viraemic transmission between vectors has also been described for tick-borne orthoflaviviruses. A new group of unclassified viruses in the genus, including cell fusing agent virus, appear only to infect mosquitoes, and several more, highly genetically distinct insect-only orthoflaviviruses have now been identified (Blitvich and Firth 2015).
Transmission
Most orthoflaviviruses are arthropod-borne viruses with cycles of transmission from hematophagous arthropod vectors to vertebrate hosts. About 50% of known orthoflaviviruses are mosquito-borne, 28% are tick-borne and the remainder transmit between rodents or between bats without known arthropod vectors. For some orthoflaviviruses, the transmission cycle has not yet been identified. In certain instances, orthoflaviviruses can be transmitted to humans by blood products, organ transplantation, non-pasteurized milk or aerosols. Some tick-borne orthoflaviviruses are known to be transmitted directly between ticks by a process known as non-viraemic transmission. In the arthropod vectors, the viruses may also be transmitted trans-ovarially or vertically (mosquitoes, ticks) and transstadially (ticks). The mechanisms of virus transmission involving the insect-only orthoflaviviruses may include vertical transmission, but other mechanisms need to be considered to explain the success with which these viruses have dispersed globally.
Geographical distribution
Orthoflaviviruses have a world-wide distribution but individual species are restricted to specific endemic or epidemic areas (e.g., yellow fever virus in tropical and subtropical regions of Africa and South America; dengue virus in tropical areas of Asia, Oceania, Africa, Australia and the Americas; Japanese encephalitis virus in Southeast Asia; tick-borne encephalitis virus in Europe and Northern Asia).
Pathogenicity
More than 50% of known orthoflaviviruses have been associated with human disease, including many important human pathogens such as yellow fever virus, dengue virus, Zika virus, Japanese encephalitis virus, West Nile virus and tick-borne encephalitis virus. The induced diseases may be associated with symptoms of the central nervous system (e.g., meningitis, encephalitis), fever, arthralgia, rash and haemorrhagic fever. Several orthoflaviviruses are pathogenic for domestic or wild animals (turkey, pig, horse, sheep, dog, grouse, muskrat) and cause economically important diseases.
Antigenicity
All orthoflaviviruses are serologically-related, which can be demonstrated by binding assays such as ELISA and by haemagglutination-inhibition using polyclonal and monoclonal antibodies. Neutralization assays are more discriminating and have been used to identify more closely related Orthoflavivirus serocomplexes (as indicated in Figure 1.Flaviviridae), although not down to the species level. The envelope protein E is the major target for neutralizing antibodies and induces protective immunity. The E protein also induces orthoflavivirus cross-reactive non-neutralizing antibodies. Antigenic sites involved in neutralization have been mapped to each of the three structural domains of the E protein. The prM and NS1 proteins can also induce antibodies that protect infected animals from lethal infection.
Species demarcation criteria
Species demarcation criteria in the genus include:
- Nucleotide and deduced amino acid sequence data.
- Antigenic characteristics.
- Geographic association.
- Vector association.
- Host association.
- Disease association.
- Ecological characteristics.
Species demarcation considers a combination of each of the criteria listed above. While nucleotide sequence relatedness and the resulting phylogenies are important criteria for species demarcation, the other listed criteria may be particularly useful in the demarcation of genetically closely related viruses. For example far-eastern (FE) strains of tick-borne encephalitis virus exhibit distinct ecological differences when compared with Omsk haemorrhagic fever virus despite the fact that they are genetically relatively closely related. FE strains of tick-borne encephalitis virus are associated predominantly with Ixodes persulcatus ticks in forest environments in far-east Russia, whereas Omsk hemorrhagic fever virus is found in the Steppe regions of western Siberia associated particularly with Dermacentor spp. and to a lesser extent with Ixodes spp. These viruses are also antigenically distinguishable in neutralization tests that employ convalescent sera.
Louping ill virus and tick-borne encephalitis virus provide another example of viruses where, despite their close genetic relationships and similar host ranges, they display different ecologies (moorlands versus forests), pathogenicities (red grouse, sheep/goats versus humans) and geographical distributions (UK versus Europe/Eurasia), thus justifying their classification as members of the distinct species, Orthoflavivirus loupingi and Orthoflavivirus encephalitidis.
On the other hand, the four dengue virus serotypes all belong to a single species (Orthoflavivirus denguei), despite being phylogenetically and antigenically quite distinct. This is justified by the fact that they co-circulate in the same geographical areas and ecological habitats, and that they exploit identical vectors, exhibit similar life cycles and disease manifestations (Table 1.Orthoflavivirus).
Table 1.Orthoflavivirus. Orthoflaviviruses grouped by vector and host.
|
Virus species |
Virus name |
Accession number |
Virus abbreviation |
|
Tick-borne, mammalian host |
|||
|
Orthoflavivirus gadgetsense |
Gadgets Gully virus |
GGYV |
|
|
Orthoflavivirus kyasanurense |
Kyasanur Forest disease virus |
KFDV |
|
|
|
Alkhumra hemorrhagic fever virus |
AHFV |
|
|
Orthoflavivirus langatense |
Langat virus |
LGTV |
|
|
Orthoflavivirus loupingi |
Louping ill virus |
LIV |
|
|
|
British subtype |
LIV-Brit |
|
|
|
Irish subtype |
LIV-Ir |
|
|
|
Spanish subtype |
LIV-Spain |
|
|
|
Turkish sheep encephalitis virus subtype |
TSEV |
|
|
|
Greek goat encephalitis virus subtype |
GGEV |
|
|
Orthoflavivirus omskense |
Omsk hemorrhagic fever virus |
OHFV |
|
|
Orthoflavivirus powassanense |
Powassan virus |
POWV |
|
|
|
deer tick virus |
DTV |
|
|
Orthoflavivirus royalense |
Royal Farm virus |
RFV |
|
|
Orthoflavivirus encephalitidis |
European subtype |
TBEV-Eur |
|
|
|
Far Eastern subtype |
TBEV-FE |
|
|
|
Siberian subtype |
TBEV-Sib |
|
|
Tick-borne, seabird host |
|||
|
Orthoflavivirus meabanense |
Meaban virus |
MEAV |
|
|
Orthoflavivirus saumarezense |
Saumarez Reef virus |
SREV |
|
|
Orthoflavivirus tyuleniyense |
Tyuleniy virus |
TYUV |
|
|
Probably tick-borne |
|
|
|
|
Orthoflavivirus kadamense |
Kadam virus |
KADV |
|
|
Mosquito-borne, Aroa virus group |
|||
|
Orthoflavivirus aroaense |
Aroa virus |
AROAV |
|
|
|
Bussuquara virus |
BSQV |
|
|
|
Iguape virus |
IGUV |
|
|
|
Naranjal virus |
NJLV |
|
|
Mosquito-borne, Dengue virus group |
|||
|
Orthoflavivirus denguei |
Dengue virus 1 |
DENV-1 |
|
|
|
Dengue virus 2 |
DENV-2 |
|
|
|
Dengue virus 3 |
DENV-3 |
|
|
|
Dengue virus 4 |
DENV-4 |
|
|
Mosquito-borne, Japanese encephalitis virus group |
|||
|
Orthoflavivirus cacipacoreense |
Cacipacoré virus |
CPCV |
|
|
Orthoflavivirus japonicum |
Japanese encephalitis virus |
JEV |
|
|
Orthoflavivirus koutangoense |
Koutango virus |
KOUV |
|
|
Orthoflavivirus murrayense |
Alfuy virus |
ALFV |
|
|
|
Murray Valley encephalitis virus |
MVEV |
|
|
Orthoflavivirus louisense |
St. Louis encephalitis virus |
SLEV |
|
|
Orthoflavivirus usutuense |
Usutu virus |
USUV |
|
|
Orthoflavivirus nilense |
Kunjin virus |
KUNV |
|
|
|
West Nile virus |
WNV |
|
|
Orthoflavivirus yaoundeense |
Yaoundé virus |
YAOV |
|
|
Mosquito-borne, Kokobera virus group |
|||
|
Orthoflavivirus kokoberaorum |
Kokobera virus |
KOKV |
|
|
|
Stratford virus |
STRV |
|
|
Mosquito-borne, Ntaya virus group |
|||
|
Orthoflavivirus bagazaense |
Bagaza virus |
BAGV |
|
|
Orthoflavivirus ilheusense |
Ilhéus virus |
ILHV |
|
|
|
Rocio virus |
ROCV |
|
|
Orthoflavivirus israelense |
Israel turkey meningoencephalitis virus |
ITV |
|
|
Orthoflavivirus ntayaense |
Ntaya virus |
NTAV |
|
|
Orthoflavivirus tembusu |
Tembusu virus |
TMUV |
|
|
Orthoflavivirus zikaense |
Zika virus |
ZIKV |
|
|
Mosquito-borne, yellow fever virus group |
|||
|
Orthoflavivirus sepikense |
Sepik virus |
SEPV |
|
|
Orthoflavivirus wesselsbronense |
Wesselsbron virus |
WESSV |
|
|
Orthoflavivirus flavi |
yellow fever virus |
YFV |
|
|
Probably mosquito-borne, Kedougou virus group |
|||
|
Orthoflavivirus kedougouense |
Kédougou virus |
KEDV |
|
|
Probably mosquito-borne, Edge Hill virus group |
|||
|
Orthoflavivirus banziense |
Banzi virus |
BANV |
|
|
Orthoflavivirus boubouiense |
Bouboui virus |
BOUV |
|
|
Orthoflavivirus edgehillense |
Edge Hill virus |
EHV |
|
|
Orthoflavivirus jugraense |
Jugra virus |
JUGV |
|
|
Orthoflavivirus saboyaense |
Potiskum virus |
POTV |
|
|
|
Saboya virus |
SABV |
|
|
Orthoflavivirus ugandaense |
Uganda S virus |
UGSV |
|
|
Unknown vector, Entebbe bat virus group |
|||
|
Orthoflavivirus entebbeense |
Entebbe bat virus |
ENTV |
|
|
|
Sokuluk virus |
SOKV |
|
|
Orthoflavivirus yokoseense |
Yokose virus |
YOKV |
|
|
Unknown vector, Modoc virus group |
|||
|
Orthoflavivirus apoiense |
Apoi virus |
APOIV |
|
|
Orthoflavivirus cowboneense |
Cowbone Ridge virus |
CRV |
|
|
Orthoflavivirus jutiapaense |
Jutiapa virus |
JUTV |
|
|
Orthoflavivirus modocense |
Modoc virus |
MODV |
|
|
Orthoflavivirus viejaense |
Sal Vieja virus |
SVV |
|
|
Orthoflavivirus perlitaense |
San Perlita virus |
SPV |
|
|
Unknown vector, Rio Bravo virus group |
|||
|
Orthoflavivirus bukalasaense |
Bukalasa bat virus |
BBV |
|
|
Orthoflavivirus careyense |
Carey Island virus |
CIV |
|
|
Orthoflavivirus dakarense |
Dakar bat virus |
DBV |
|
|
Orthoflavivirus montanaense |
Montana myotis leukoencephalitis virus |
MMLV |
|
|
Orthoflavivirus phnompenhense |
Batu Cave virus |
BCV |
|
|
|
Phnom Penh bat virus |
PPBV |
|
|
Orthoflavivirus bravoense |
Rio Bravo virus |
RBV |
|
Member species
The Member Species table enumerating important virus exemplars classified under each species of the genus is provided at the bottom of the page.
Related, unclassified viruses
|
Virus name |
Accession number |
Virus abbreviation |
|
Mammalian tick-borne |
||
|
Karshi virus |
KSIV |
|
|
Mosquito-borne |
||
|
Fitzroy River virus |
FRV | |
|
Spondweni virus |
SPOV |
|
|
T’Ho virus |
|
|
|
Insect-specific orthoflaviviruses |
||
|
Aedes flavivirus |
AEFV |
|
|
Aedes galloisi flavivirus |
|
AGFV |
|
Anopheles flavivirus |
AnFV |
|
|
Calbertado virus |
CLBOV |
|
|
cell fusing agent virus |
CFAV |
|
|
Cuacua virus |
CuCuV |
|
|
Culex flavivirus |
CXFV |
|
|
Culex theileri flavivirus |
CXthFV |
|
|
Culiseta flavivirus |
CsFV |
|
|
Ecuador Paraiso Escondido virus |
EPEV |
|
|
Hanko virus |
HaFV |
|
|
Kamiti River virus |
KRV |
|
|
Mercadeo virus |
MECDV |
|
|
mosquito flavivirus |
MoFV |
|
|
Nakiwogo virus |
NAKV |
|
|
Nienokoue virus |
NiFV |
|
|
Palm Creek virus |
PCFV |
|
|
Parramatta River virus |
PaRV |
|
|
Quảng Bình virus |
QBV |
|
|
Xishuangbanna aedes flavivirus |
XFV |
|
|
Yamadai flavivirus |
YDFV |
|
|
Viruses with no known arthropod vector |
||
|
Barkedji virus |
BJV |
|
|
Cháoyáng virus |
CHAOV |
|
|
Donggang virus |
DONV |
|
|
Ilomantsi virus |
ILOV |
|
|
Kampung Karu virus |
KPKV |
|
|
Lammi virus |
LAMV |
|
|
La Tina virus |
LTNV |
|
|
Long Pine Key virus |
LPKV |
|
|
Marisma mosquito virus |
MMV |
|
|
Nanay virus |
NANV |
|
|
Ngoye virus |
NGOV |
|
|
Nhumirim virus |
NHUV |
|
|
nounané virus |
NOUV |
|
|
Tamana bat virus |
TABV |
|
|
Segmented flavi-like viruses |
||
|
Jingmen tick virus |
JMTV |
|
|
Mogiana tick virus |
MGTV |
|
|
Alongshan virus |
ASV |
|
|
Guaico Culex virus |
GCXV |
|
|
Shuangao insect virus 7 |
SAIV7 |
|
|
Wuhan flea virus |
WHFV |
|
|
Wuhan aphid virus 1 |
WHAV1 |
|
|
Wuhan aphid virus 2 |
WHAV2 |
|
|
Wuhan cricket virus |
WHCV |
Virus names and virus abbreviations are not official ICTV designations.