Family: Baculoviridae
Genus: Alphabaculovirus
Distinguishing features
Occlusion bodies typically measure 0.5 to 5 µm in size and are generally polyhedral, although morphology may vary and size may reach 15 µm (Adams and McClintock 1991). They mature within the nuclei of infected cells and characteristically contain many enveloped virions (Figure 1.Alphabaculovirus). The occluded virions are packaged with either single or multiple nucleocapsids within a single viral envelope. Members of some virus species manifest both phenotypes. A number of genes that influence nucleocapsid packaging are known, but in members of some species, packaging may be variable (Rohrmann 2014, Yang et al., 2014). Single (S) and multiple (M) designations in common names have been retained for species where variability has not been reported and for distinct viruses that would otherwise have identical designations under the current nomenclature. During viral entry, nucleocapsids are transported through the nuclear membrane and into the nucleus (Au et al., 2016), where uncoating and viral replication occur.
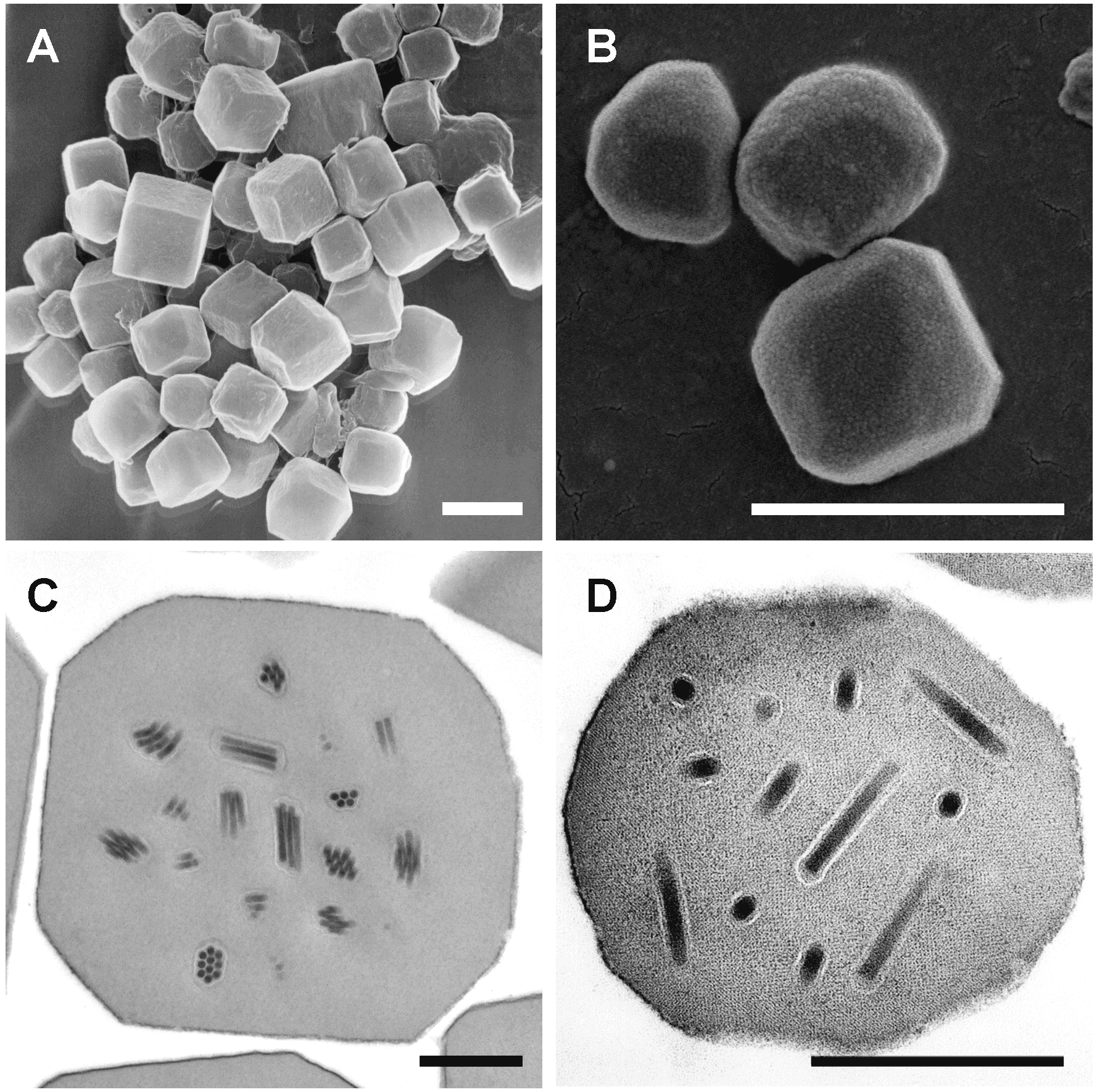 |
| Figure 1. Alphabaculovirus. Occlusion bodies of baculoviruses in the genus Alphabaculovirus. Scanning electron micrographs of Autographa californica multiple nucleopolyhedrovirus (A) and Operophtera brumata nucleopolyhedrovirus (B) occlusion bodies are shown, along with transmission electron micrographs of Autographa californica multiple nucleopolyhedrovirus (C) and Trichoplusia ni single nucleopolyhedrovirus (D) occlusion bodies containing occlusion-derived virus consisting of multiple and single nucleocapsids per envelope, respectively. Scale bars: (A, B) 2 µm; (C, D) 0.5 µm. Micrographs in panels (A) and (D) courtesy of J. R. Adams (USDA). |
Virion
Morphology
See family description. Nucleocapsid length appears to be proportional to genome size (Fraser 1986).
Genome organization and replication
In addition to the family Baculoviridae core genes, the alphabaculoviruses and betabaculoviruses appear to share an additional 23 homologs (Garavaglia et al., 2012).
Biology
Species of this genus have been isolated only from the insect order Lepidoptera.
Species demarcation criteria
Traditionally, species distinctions have been broadly based on host range and specificity, DNA restriction profiles, DNA sequences from various regions of the genome, and predicted protein sequence similarities. More recently, species demarcation criteria for alpha- and betabaculoviruses have been proposed that rely upon pairwise nucleotide distances estimated with the Kimura-2-parameter substitution model from partial sequences of three conserved baculovirus genes: late expression factor-8 (lef-8) and late expression factor-9 (lef-9), which encode subunits of the baculovirus RNA polymerase, and polyhedrin/granulin (polh/gran), which encodes the viral occlusion body matrix protein (Jehle et al., 2006b). If nucleotide distances between two viruses at these loci are less than 0.015 substitutions/site, the two baculoviruses are considered to belong to the same species. If nucleotide distances between two viruses are greater than 0.05 substitutions/site, the viruses are considered to belong to different species. If the nucleotide distances lie between 0.015 and 0.050 substitutions/site, additional characteristics of the two viruses (i.e. host range) must be considered to make a decision about their taxonomic status. The proposed criteria were originally based on an alignment of sequences from 117 separate baculovirus isolates and the phylogeny inferred from this alignment. Researchers have applied this criterion to other isolates to identify many new baculovirus species and variants of currently recognized species. A more recent examination of pairwise nucleotide distances for all 38 baculovirus core genes among 172 completely sequenced baculovirus genomes has confirmed the current species classification based on pairwise distances for lef-8, lef-9, and polh loci (Wennmann et al., 2018).
Related, unclassified viruses
| Virus name | Accession number | Virus abbreviation |
| Apocheima cinerarium nucleopolyhedrovirus | FJ914221 | ApciNPV |
| Cerapteryx graminis nucleopolyhedrovirus | HQ603182; HQ603183; HQ603184 | CegrNPV |
| Dasychira pudibunda nucleopolyhedrovirus | KP747440 | DapuNPV-ML1 |
| Lacanobia oleracea nucleopolyhedrovirus | LaolNPV | |
| Leucoma salicis multiple nucleopolyhedrovirus | AY729808; AY729809; AY729810 | LesaMNPV |
| Malacosoma californicum pluviale nucleopolyhedrovirus | AF535137 | McplNPV |
| Malacosoma neustria nucleopolyhedrovirus | KY968317 | ManeNPV-T2 |
| Operophtera bruceata nucleopolyhedrovirus | KY064005; KY064007 | OpbrNPV |
| Orgyia pseudotsugata single nucleopolyhedrovirus | MZ127289 | OpSNPV-MEM |
| Panolis flammea nucleopolyhedrovirus | D00437 | PaflNPV |
| Philosamiacynthia nucleopolyhedrovirus | JX404026 | PhcyNPV |
| Platynota idaeusalis nucleopolyhedrovirus | OQ658191 | PlidNPV-2680 |
| Spodoptera litura nucleopolyhedrovirus II | EU780426 | SpltNPV-II |
| Troides aeacus nucleopolyhedrovirus | MH077961 | TraeNPV-wt |
Virus names and virus abbreviations are not official ICTV designations.

