Family: Baculoviridae
Robert L. Harrison, Elisabeth A. Herniou, Johannes A. Jehle, David A. Theilmann, John P. Burand, Peter J. Krell, Monique M. van Oers and Joseph D. Mowery
The citation for this ICTV Report chapter is the summary published as Harrison et al., (2019):
ICTV Virus Taxonomy Profile: Baculoviridae, Journal of General Virology, 2019; 99: 1185–1186.
Corresponding author: Monique M. van Oers (monique.vanoers@wur.nl)
Edited by: Balázs Harrach and Andrew J. Davison
Posted: June 2018, revised October 2021, April 2022 and December 2023
PDF: ICTV_Baculoviridae.pdf (October 2020 version)
Summary
Baculoviridae is a family of large, insect-specific viruses with circular dsDNA genomes ranging from 80 to 180 kbp (Table 1.Baculoviridae). Virions consist of enveloped, rod-shaped nucleocapsids and are embedded in distinctive occlusion bodies typically measuring 0.5–5 µm. The occlusion bodies consist of a matrix composed of a single viral protein expressed at high levels during infection. Members of this family infect exclusively larvae of insect orders Lepidoptera, Hymenoptera and Diptera. Some members have been developed as biopesticides for controlling insect pests and as vectors for recombinant protein expression. Further reading: (Miller 1997, Herniou et al., 2003, Braunagel and Summers 2007, Herniou and Jehle 2007, van Oers and Vlak 2007).
Table 1. Baculoviridae. Characteristics of members of the family Baculoviridae.
| Characteristic | Description |
| Example | Autographa californica multiple nucleopolyhedrovirus C6 (L22858), species Alphabaculovirus aucalifornicae, genus Alphabaculovirus |
| Virion | One or two distinct types of virions consisting of enveloped, rod-shaped nucleocapsids, 30–60 nm × 250–300 nm, containing >20 proteins |
| Genome | A single covalently-closed circular dsDNA molecule of 80–180 kbp encoding 100–200 proteins |
| Replication | Nuclear, with nucleocapsids assembled in the nucleus and enveloped either (a) in the nucleus or mixed nucleoplasm and cytoplasm, or (b) upon budding through the plasma membrane |
| Translation | From mRNAs transcribed from viral DNA |
| Host range | Larval-stage insects of orders Diptera, Hymenoptera, and Lepidoptera |
| Taxonomy | Class Naldaviricetes, order Lefavirales; the family has four genera with 101 species |
Virion
Morphology
One or two virion phenotypes are involved in baculovirus infections. Infection is initiated in the gut epithelium by virions contained within a crystalline protein occlusion body (OB) which may be polyhedral in shape and containing many virions (members of the genera Alphabaculovirus, Gammabaculovirus and Deltabaculovirus); or ovocylindrical and containing only one, or rarely two or more, virions (members of the genus Betabaculovirus). Virions within occlusions, referred to as occlusion-derived virus (ODV), consist of one or more rod-shaped nucleocapsids that have a distinct structural polarity and are enclosed within an envelope (Figure 1.Baculoviridae). For ODV, nucleocapsid envelopment occurs within the nucleus (members of the genus Alphabaculovirus) or in the nuclear-cytoplasmic milieu after loss of the nuclear membrane (members of the genus Betabaculovirus). Nucleocapsids average 30–60 nm in diameter and 250–300 nm in length. Spike-like structures (peplomers) have not been reported on envelopes of ODV. Virions of the second phenotype (termed budded virus or BV; Figure 1.Baculoviridae) are generated when nucleocapsids bud through the plasma membrane at the surface of infected cells. BVs typically contain a single nucleocapsid. Their envelopes are derived from the cellular plasma membrane and characteristically appear as a loose-fitting membrane that contains an envelope fusion glycoprotein (EFP), which forms peplomers usually observed at one or both ends of the virion ((Wang et al., 2016); Figure 1.Baculoviridae; see “Proteins” below).
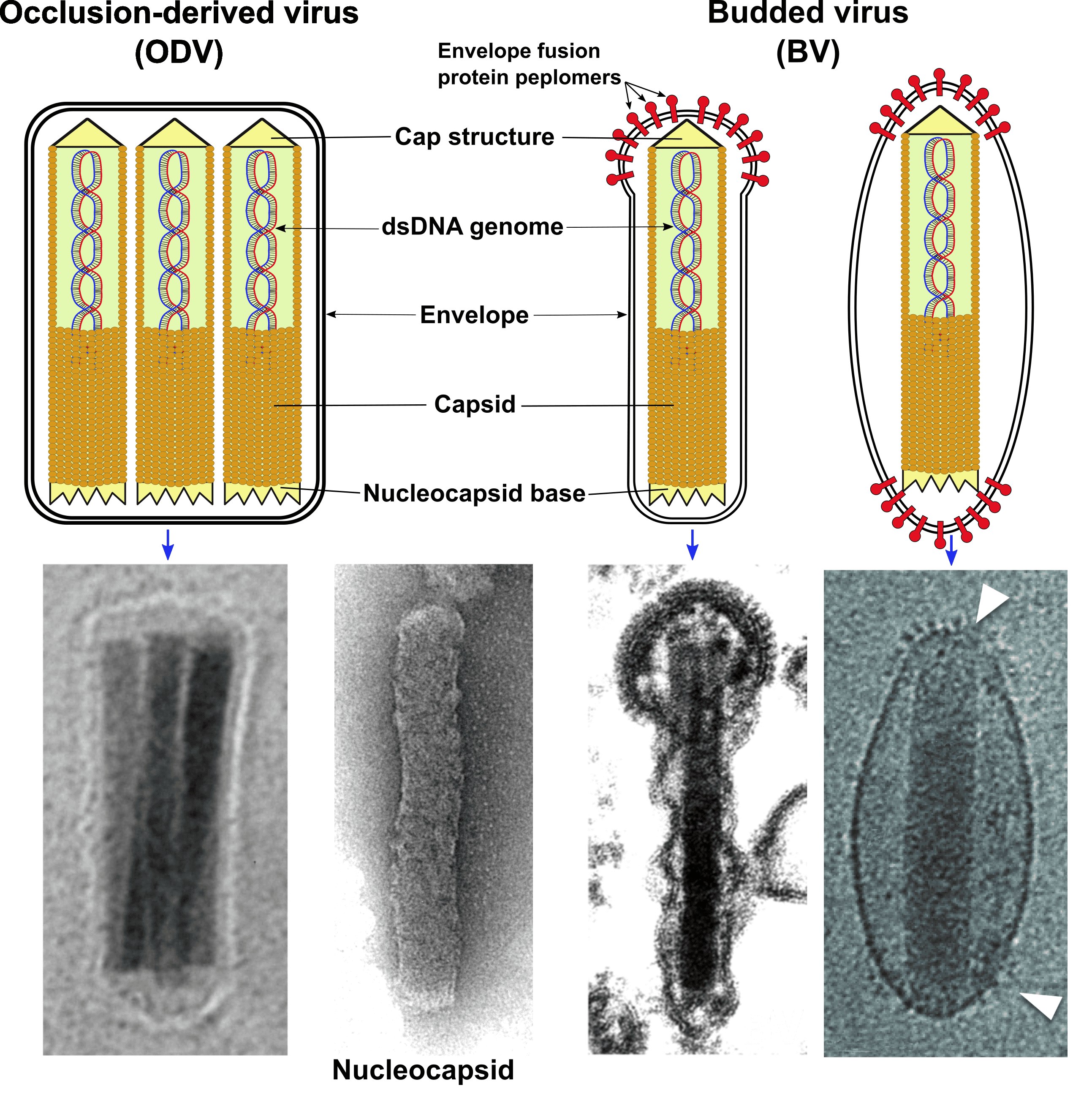 |
| Figure 1. Baculoviridae. Baculovirus virions and nucleocapsids. (Top) The two baculovirus virion phenotypes are illustrated as diagrams with shared and phenotype-specific components. (Bottom) Electron micrographs of an occlusion-derived virion of Autographa californica multiple nucleopolyhedrovirus (far left), a negatively-stained nucleocapsid of Autographa californica multiple nucleopolyhedrovirus (center left), a budded virion of Lymantria dispar multiple nucleopolyhedrovirus (center right), and a budded virion of Spodoptera exigua multiple nucleopolyhedrovirus (far right). Nucleocapsid and Lymantria dispar multiple nucleopolyhedrovirus budded virion micrographs courtesy of J. R. Adams (USDA). Spodoptera exigua multiple nucleopolyhedrovirus micrograph reprinted from ((Wang et al., 2016) with permission from Elsevier (http://www.elsevier.com). |
Physicochemical and physical properties
ODV buoyant density in CsCl is 1.18–1.25 g cm−3, and that of the nucleocapsid is 1.47 g cm−3. BV buoyant density in sucrose is 1.17–1.18 g cm−3 (Summers and Volkman 1976). Virions of both phenotypes are sensitive to organic solvents and detergents. ODV and BV are marginally sensitive to heat and inactivated at extreme pH values (Gudauskas and Canerday 1968, Knittel and Fairbrother 1987).
Nucleic acid
Nucleocapsids contain one molecule of circular, supercoiled dsDNA of 80–180 kbp (Figures 1.Baculoviridae, 2.Baculoviridae).
Proteins
Genomic analyses suggest that baculoviruses encode approximately 100–200 proteins. Proteomics analyses to date indicate that virions may contain as few as 23 and as many as 73 different polypeptides (Deng et al., 2007, Zhang et al., 2015). Nucleocapsids from both virion phenotypes (ODV and BV) contain a major capsid protein (VP39), a basic DNA-binding protein (P6.9) complexed with the viral genome, and at least 2–3 additional proteins. Two different EFPs have been identified to date. The EFP GP64 is present in a group of alphabaculoviruses that include Autographa californica multiple nucleopolyhedrovirus (AcMNPV) and close relatives (Blissard and Wenz 1992). Most of the alphabaculoviruses and betabaculoviruses encode and appear to utilize EFPs known as F proteins, which are homologs of the Ld130 protein from Lymantria dispar multiple nucleopolyhedrovirus (LdMNPV) (Pearson et al., 2001) and the Se8 protein from Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) isolate US1 (IJkel et al., 2000). The deltabaculovirus Culex nigripalpus nucleopolyhedrovirus also encodes an EFP with fusogenic activity (Wang et al., 2017a). Several ODV envelope proteins have been identified. Ten ODV proteins, collectively referred to as per os infectivity factors (PIFs), are essential for oral infectivity of ODV (Wang et al., 2017b, Boogaard et al., 2018, Boogaard et al., 2019). The major protein of the OB matrix is a virus-encoded polypeptide of 25–33 kDa. This protein is called polyhedrin for nucleopolyhedroviruses (the common name used for the alpha-, delta- and gammabaculoviruses) and granulin for granuloviruses (betabaculoviruses) (Rohrmann 1986). The OB is often surrounded by an envelope that contains at least one major protein (Whitt and Manning 1988). The polyhedrin protein of deltabaculoviruses is serologically and genetically unrelated to OB proteins of the alpha-, beta- and gammabaculoviruses (Perera et al., 2006).
Lipids
Lipids are present in the envelopes of ODV and BV. Lipid composition differs between the two virion phenotypes (Braunagel and Summers 1994).
Carbohydrates
Carbohydrates are present as glycoproteins and glycolipids.
Genome organization and replication
Circular genomic DNA is infectious, suggesting that after cellular entry and uncoating, no virion-associated proteins are essential for infection (Burand et al., 1980, Carstens et al., 1980). Thirty-eight gene homologs are shared among alpha-, beta, gamma- and deltabaculoviruses (Garavaglia et al., 2012, Javed et al., 2017), and are hence, regarded as the baculovirus core genes (Figure 2.Baculoviridae). These conserved genes are involved in various functions, including DNA replication, late gene transcription and virion structure. In some cases, larger genome sizes may result from the presence of families of repeated genes (Hayakawa et al., 1999). Transcription of baculovirus genes is temporally regulated, and two main classes of genes are recognized: early and late. Late genes may be further subdivided into late and very late genes. Gene classes (early, late and very late) are not clustered on the baculovirus genome, and both strands of the genome are involved in coding functions. Early genes are transcribed by host RNA polymerase II, whereas late and very late genes are transcribed by an alpha-amanitin-resistant viral RNA polymerase (Huh and Weaver 1990). RNA splicing occurs, but appears to be rare (Chisholm and Henner 1988, Pearson and Rohrmann 1997). Transient early and late gene transcription and DNA replication studies suggest that at least three virus-encoded proteins regulate early gene transcription (Guarino and Summers 1986, Kovács et al., 1991, Lu and Carstens 1993, Yoo and Guarino 1994), whereas approximately 20 viral encoded proteins known as late expression factors (LEFs) are necessary for late gene transcription (Rapp et al., 1998, Huijskens et al., 2004). Of the approximately 20 LEFs, half appear to be involved in DNA replication (Lu and Miller 1995). Late gene transcription initiates at the second adenine of a conserved 5′-TAAG-3′ promoter motif, which is an essential core element of the baculovirus late promoter (Chen et al., 2013). Putative replication origins consist of repeated sequences found at multiple locations within the baculovirus genome (Pearson et al., 1992, Hilton and Winstanley 2007). These sequences, termed homologous repeat (hr) regions, do not appear to be highly conserved among different baculovirus species. Single copy, non-hr putative replication origins have also been identified (Kool et al., 1994). DNA replication is required for late gene transcription. Most virion structural proteins are encoded by late genes. While transcription of late and very late genes appears to begin immediately after DNA replication, some very late genes that encode occlusion body-specific proteins are transcribed at extremely high levels at a later time (Thiem and Miller 1990). BV production occurs primarily during the late phase, and occlusion body production occurs during the very late phase.
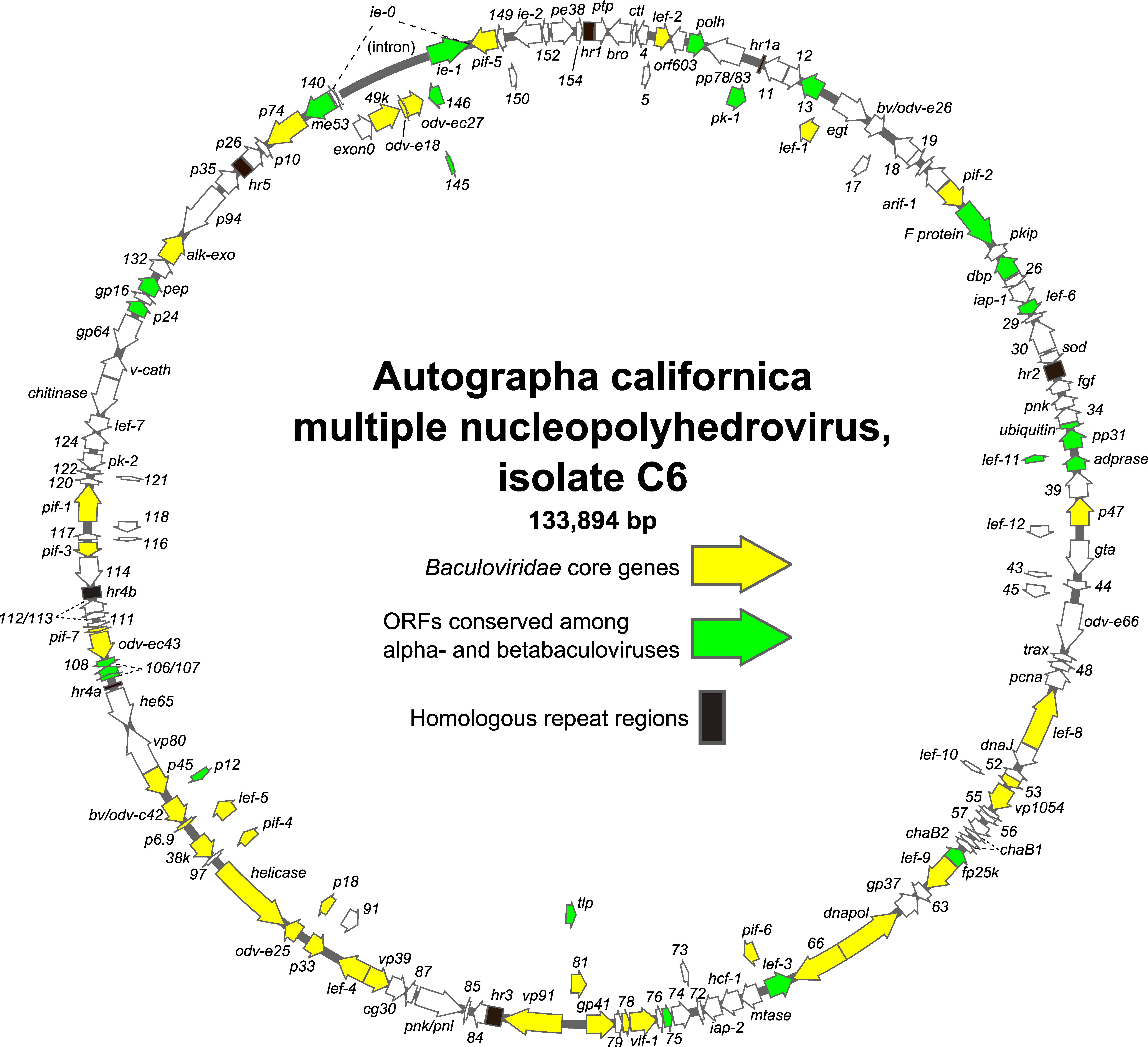 |
| Figure 2.Baculoviridae. Genome map of Autographa californica multiple nucleopolyhedrovirus isolate C6, the representative isolate of the genus Alphabaculovirus, species Alphabaculovirus aucalifornicae. The genome is illustrated with locations and orientations of annotated ORFs (arrows). ORFs corresponding to the core genes of members of the family Baculoviridae, and ORFs conserved among alpha-and betabaculoviruses are indicated. Locations of homologous repeat (hr) sequences are also shown, as well as the exons and intron of the ie-0 transcription unit. |
In infected animals, viral replication begins in the insect midgut. Following ingestion, OBs are solubilized in the gut lumen, releasing the ODVs, which are thought to enter the target epithelial cells via fusion with the plasma membrane at the cell surface (Kawanishi et al., 1972). In lepidopteran insects, viral entry into midgut cells occurs in an alkaline environment, up to pH 12, and is mediated by a complex of PIF envelope proteins. (See “Proteins” above). Infection of the midgut is required for initiation of infection in the animal. Although the virus is believed to undergo one round of replication in the midgut epithelium prior to transmission of infection to secondary tissues within the hemocoel, a mechanism for direct movement from the midgut to the hemocoel has also been proposed (Granados and Lawler 1981, Washburn et al., 1999, Washburn et al., 2003).
DNA replication takes place in the nucleus. In betabaculovirus-infected cells, the integrity of the nuclear membrane is lost during the replication process (Walker et al., 1982, Federici and Stern 1990). With some baculoviruses, replication is restricted to the gut epithelium and progeny virions become enveloped and occluded within these cells, and may be shed into the gut lumen with sloughed epithelium, or released upon death of the host (Federici and Stern 1990). In other baculoviruses, the infection is transmitted to internal organs and tissues (Keddie et al., 1989). The second virion phenotype, BV, which buds from the basolateral membrane of infected gut cells, is required for transmission of the infection into the hemocoel. Infected fat body cells are the primary location of occluded virus production in lepidopteran insects. Occluded virus matures within nuclei of infected cells for alpha-, gamma- and deltabaculoviruses, and within the nuclear-cytoplasmic milieu for betabaculoviruses. OBs containing infectious ODV virions are released upon death, and usually liquefaction, of the host.
Biology
- Baculoviruses have been isolated from insects only - primarily from insects of the order Lepidoptera, but also from the orders Hymenoptera and Diptera. Transmission naturally occurs (i) horizontally by contamination with OBs of food, egg surfaces, etc. (Hamm and Young 1974, Young and Yearian 1986); (ii) vertically within the egg either from infected female or male adults (Doane 1969); or experimentally; (iii) by injection of intact hosts with BVs; or (iv) by infection or transfection of cell cultures. Typically, the infection process in a permissive insect host requires approximately one week, and, as an end result, the diseased insect liquefies, releasing infectious occlusion bodies into the environment. OBs represent an environmentally stable form of the virus with increased resistance to chemical and physical decay as well as inactivation by UV light. Persistent, asymptomatic infections have also been documented (Myers and Cory 2016).
Antigenicity
Antigenic determinants that cross-react between different baculoviruses exist on virion proteins and on the major OB polypeptide: polyhedrin or granulin (Summers et al., 1978, Volkman 1983). Neutralizing antibodies react with the major surface glycoprotein of BVs (Volkman et al., 1984).
Derivation of names
Alphabaculovirus, Betabaculovirus, Gammabaculovirus, Deltabaculovirus: Greek letters α, β, γ, and δ, the first four letters of the Greek alphabet.
Baculoviridae: from baculum, meaning “rod” in Latin, referring to the morphology of the nucleocapsid.
granulovirus: used as part of virus isolate names for members of the genus Betabaculovirus and derived from “granule” and “granulosis”, referring to the relatively small size of OBs and their granular appearance in betabaculovirus-infected cells.
nucleopolyhedrovirus: used as part of names for virus isolates in genera apart from Betabaculovirus and derived from “nuclear polyhedrosis” and “polyhedron”, referring to the multifaceted appearance of OBs in the nuclei of infected cells.
Baculovirus species names were converted to a binomial format in 2023, where the species epithet is derived from the name of the host of the exemplar virus isolate (van Oers et al., 2023).
Genus demarcation criteria
Genera of Baculoviridae are distinguished on the basis of phylogeny, genome characteristics (especially gene content), host range and OB morphology (Jehle et al., 2006a).
Relationships within the family
Phylogenetic analysis based on the 38 baculovirus core genes shows that the family comprises four monophyletic groups (Figure 3.Baculoviridae), which can also be discriminated based on the orders of their insect hosts and on their morphology. Hence, baculoviruses are classified into the four genera Alphabaculovirus, Betabaculovirus, Gammabaculovirus and Deltabaculovirus.
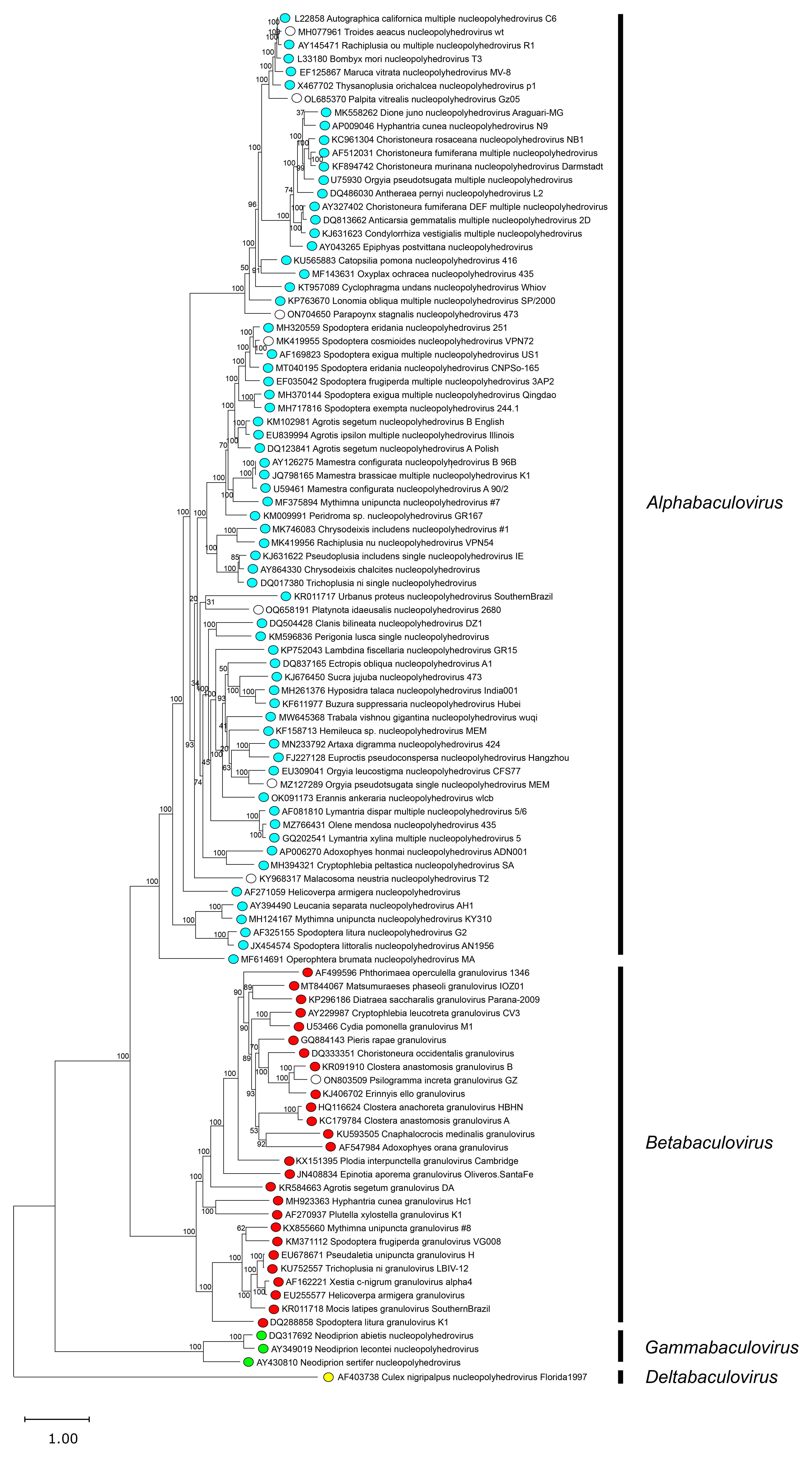 |
| Figure 3. Baculoviridae. Phylogeny of family Baculoviridae. The maximum likelihood tree, based on the alignment of 38 core gene amino acid sequences, shows the relationships of the species for which a completely annotated genome of a representative isolate was available at time of analysis. Amino acid sequences of the 38 core genes were aligned individually by MUSCLE and concatenated, and phylogeny was inferred from the concatenated alignments with the Le and Gascuel (LG) substitution model using RAxML with 100 bootstrap replicates. Colored circles indicate genus assignments for each virus; unclassified viruses are indicated by open circles. This phylogenetic tree and corresponding sequence alignment are available to download from the Resources page. |
Relationships with other taxa
Members of the family Baculoviridae share structural, genetic and biological characters with viruses of the family Nudiviridae, which had been formerly classified as “non-occluded” baculoviruses. The nudiviruses share at least 20 core genes with baculoviruses (Wang et al., 2011). Baculoviruses also share characteristics with salivary gland hypertrophy viruses in the family Hytrosaviridae, which possess at least 12 baculovirus core genes, and to a lesser extent to the white spot syndrome virus of the family Nimaviridae (Jehle et al., 2013). The families Baculoviridae, Nudiviridae, Hytrosaviridae, and Nimaviridae have been grouped into the class, Naldaviricetes, with three of these families (Baculoviridae, Nudiviridae, and Hytrosaviridae) organized into the subordinate order, Lefavirales. An important discriminative trait carried by all viruses in the class Naldaviricetes is the presence of pif-genes, encoding per os infectifity factors (PIF proteins).

