Family: Nudiviridae
Genus: Betanudivirus
Distinguishing features
The genus Betanudivirus currently includes a single species (Betanudivirus hezeae) with two well-characterized isolates from either cells or insects of the noctuid moth Helicoverpa (formerly Heliothis) zea. Virions are elongated relative to alphanudivirus virions, measuring 414–437 nm × 80–93 nm (Granados et al., 1978, Burand and Lu 1997) (Figure 1.Betanudivirus). Isolate Heliothis zea nudivirus 1 (HzNV-1) was originally discovered covertly infecting the moth cell line IMC-Hz-1, derived from ovaries of Helicoverpa zea (order Lepidoptera), and was found to be incapable of infecting larvae (Granados et al., 1978). Helicoverpa zea nudivirus 2 (HzNV-2) was originally identified as a virus associated with gonadal atrophy in adult moths of Helicoverpa zea (Raina and Adams 1995). Unlike HzNV-1, HzNV-2 can infect both larvae and cell lines (Raina and Adams 1995, Burand and Lu 1997). It has been speculated that the IMC-Hz-1 cell line in which HzNV-1 was originally discovered was developed with ovarian tissue from insects infected with HzNV-2.
 |
|
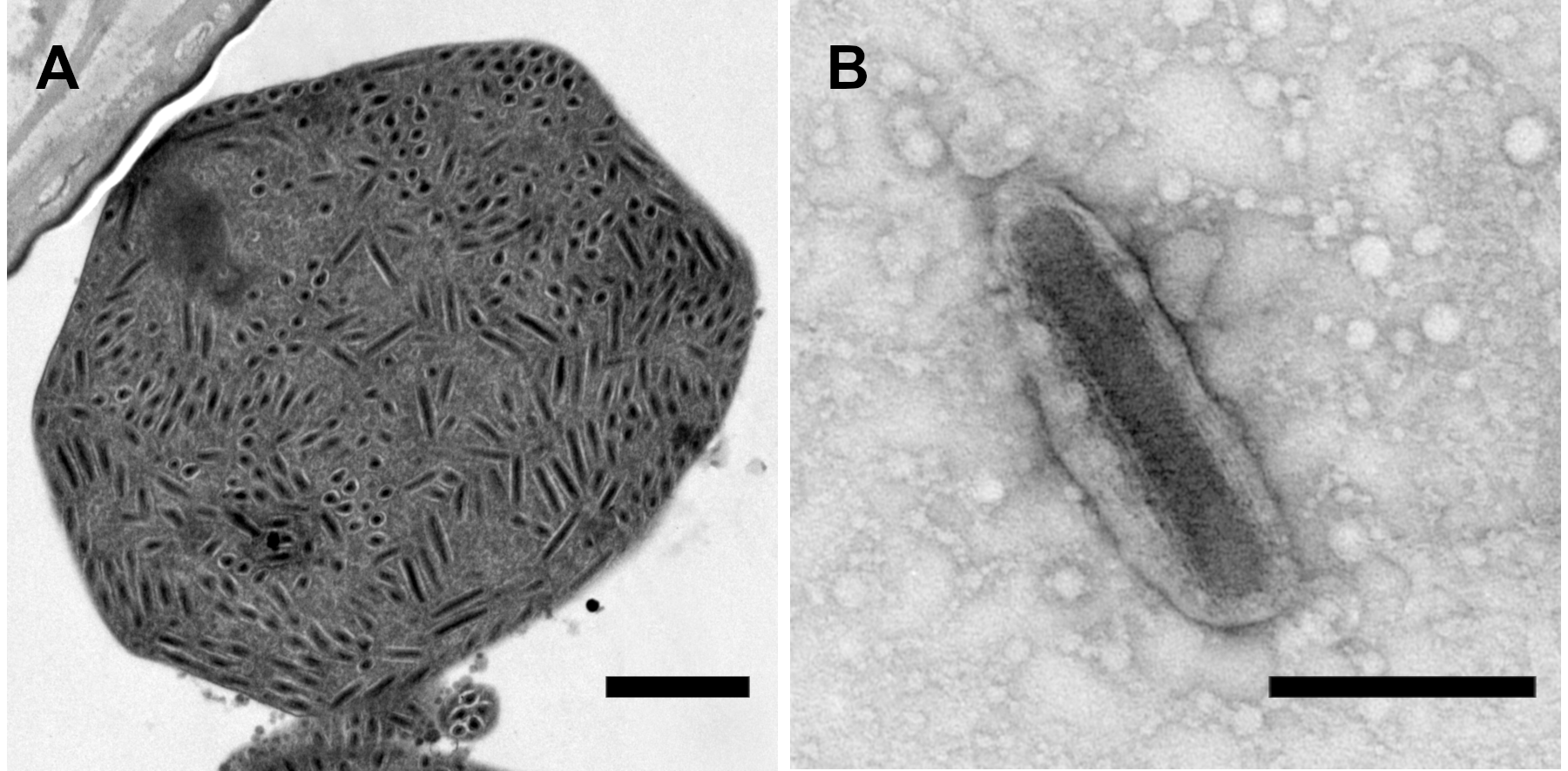
Figure 1.Betanudivirus. (A) A cluster of virions in the bursa copulatrix of an adult female H. zea moth infected with Helicoverpa zea nudivirus 2 (HzNV-2). Virions appear to be embedded in a granular matrix material, resulting in an occlusion body with an atypical appearance. (B) HzNV-2 virion derived from an atypical occlusion body treated with sodium carbonate. Scale bars: (A) 1 µm; (B) 0.25 µm. Micrographs produced by J. R. Adams (USDA). |
Genome organization and replication
The genomes of HzNV-1 and HzNV-2 are 228 089 and 231 621 bp in size, respectively, with a GC content of 41.8–41.9% GC (Cheng et al., 2002, Burand et al., 2012). ORFs annotated for these genome sequences numbered 154 for HzNV-1 and 113 for HzNV-2, a difference that seems to be due in part to differences in the criteria used for ORF annotation. Initial infection of cells by HzNV-1 is characterized by active viral replication and release of progeny virus upon cell lysis. Few cells survive the infection and these continue to harbour HzNV-1 in a latent state (Granados et al., 1978, Ralston et al., 1981). The conversion of an active HzNV-1 infection to a persistent, asymptomatic infection is mediated by two microRNAs (miRNAs) that appear to be derived from the transcript of the HzNV-1 persistency-associated gene 1 (pag1) (Wu et al., 2011), which is expressed during the initial active infection and in persistently infected cells. The pag1-encoded miRNAs reduce the steady-state levels of the transcript of the HzNV-1 hhi1 gene, which is transcribed early after infection and can stimulate the expression of other HzNV-1 early genes (Wu et al., 2010).
HzNV-2 replication in tissue culture cells also results in cell lysis and release of progeny virions (Burand and Lu 1997), but infections of insects generally do not result in mortality. HzNV-2 progeny virions can be observed in the deformed reproductive tissues of adults with gonadal atrophy that have developed from infected larvae, and masses of virions present in vesicles or in structures that superficially resemble the occlusion bodies of other large DNA insect viruses have been reported in the oviducts and bursa copulatrix of agonadal adult females (Figure 1.Betanudivirus) (Hamm et al., 1996, Raina et al., 2000, Rallis and Burand 2002a, Rallis and Burand 2002b). The virions in this coalesced form make up a waxy plug that covers the reproductive opening at the tip of the abdomen in agonadal females.
Biology
Both HzNV-1 and HzNV-2 can infect cells derived from several heterologous host species (Burand and Lu 1997, McIntosh et al., 2007). HzNV-2 has been detected in wild and laboratory populations of only H. zea (Lupiani et al., 1999), and replication appears to be limited to reproductive tissues. Larvae infected with HzNV-2 exhibit no obvious pathology but can develop into adults with either virus-caused gonadal atrophy or a persistent, asymptomatic infection. Sexual contact appears to be a primary route of transmission for HzNV-2. Infected agonadal male and female moths can transmit the virus to uninfected adults by mating. A female moth that has been infected by mating with an agonadal male can subsequently vertically transmit the virus to the progeny resulting from mating with a fertile (uninfected) male (Hamm et al., 1996). Similarly, a male moth acquiring the virus by mating with an infected agonadal female can transmit the virus to progeny arising from mating with an uninfected female (Burand et al., 2004). Female moths infected as larvae that have developed asymptomatic infections can also transmit the virus to their progeny. Larvae and adults can also be infected by direct injection of virus.
Species demarcation criteria
Not defined as there is currently only one species in the genus.

