Family: Polyomaviridae
Ugo Moens, Sébastien Calvignac-Spencer, Chris Lauber, Torbjörn Ramqvist, Mariet C. W. Feltkamp, Matthew D. Daugherty, Ernst J. Verschoor and Bernhard Ehlers
The citation for this ICTV Report chapter is the summary published as Moens et al., (2017):
ICTV Virus Taxonomy Profile: Polyomaviridae, Journal of General Virology, 98: 1159–1160
Corresponding author: Sébastien Calvignac-Spencer (calvignacs@rki.de)
Edited by: Balázs Harrach and Andrew J. Davison
Posted: June 2017, updated July 2018, February 2020 and September 2021
PDF: ICTV_Polyomaviridae.pdf (of 2020 version)
Summary
Polyomaviridae is a family of small, non-enveloped viruses with dsDNA genomes of approximately 5,000 base pairs (Table 1. Polyomaviridae). Phylogenetic relationships among polyomaviruses, based on the amino acid sequence of the viral protein large tumor antigen, have resulted in the delineation of 117 species, 112 of which have been assigned to 6 genera: Alphapolyomavirus, Betapolyomavirus, Gammapolyomavirus, Deltapolyomavirus, Epsilonpolyomavirus and Zetapolyomavirus. The members of these genera can infect mammals and birds, and polyomavirus genomes have recently been detected in fish. Each family member has a restricted host range. Some members are known human and veterinary pathogens causing symptomatic infection or cancer in their natural host. Clinical manifestations are observed primarily in immunocompromised patients.
Table 1. Polyomaviridae. Characteristics of members of the family Polyomaviridae.
| Characteristic | Description |
| Example | Simian virus 40 (J02400), species Betapolyomavirus macacae |
| Virion | Non-enveloped, 40–45 nm, icosahedral |
| Genome | Approximately 5 kbp circular dsDNA |
| Replication | Bidirectional from an unique origin of replication |
| Translation | Early and late transcripts, alternative splicing, alternative ORFs |
| Host Range | Mammals, birds and fish |
| Taxonomy | Realm Monodnaviria, kingdom Shotokuvirae, phylum Cossaviricota, class Papovaviricetes, order Sepolyvirales; 8 genera containing 122 species |
Virion
Morphology
Mature virions measure approximately 40–45 nm in diameter and consist of 88% protein and 12% DNA. VP1 is the major protein and accounts for 75% of the total virion protein mass. For most mammalian polyomaviruses, VP2 and VP3 are minor capsid proteins, and bird polyomaviruses have an additional unique VP4. The virions are non-enveloped with a capsid that has a T=7dextro (right-handed) icosahedral symmetry and is made up of 72 pentameric capsomers (Figure 1. Polyomaviridae). Each pentamer is composed of five VP1 molecules. The capsomers are interlinked by the C-terminal arm of VP1. Capsomer contacts are further stabilized by calcium ions and disulfide bonds between the pentamers. A single copy of VP2 or VP3 binds in a hairpin manner into the cavity on the internal face of each pentamer. The VP4 of bird polyomaviruses is located between VP1 and the viral genome. Each virion contains a single copy of a circular dsDNA. The mature viral genome is organized as a minichromosome packed with histone proteins H2A, H2B, H3 and H4. The VP1 N-terminus is bound to the packaged DNA, but VP2/3 can also be associated with the viral genome (Hurdiss et al., 2016, Shen et al., 2011).
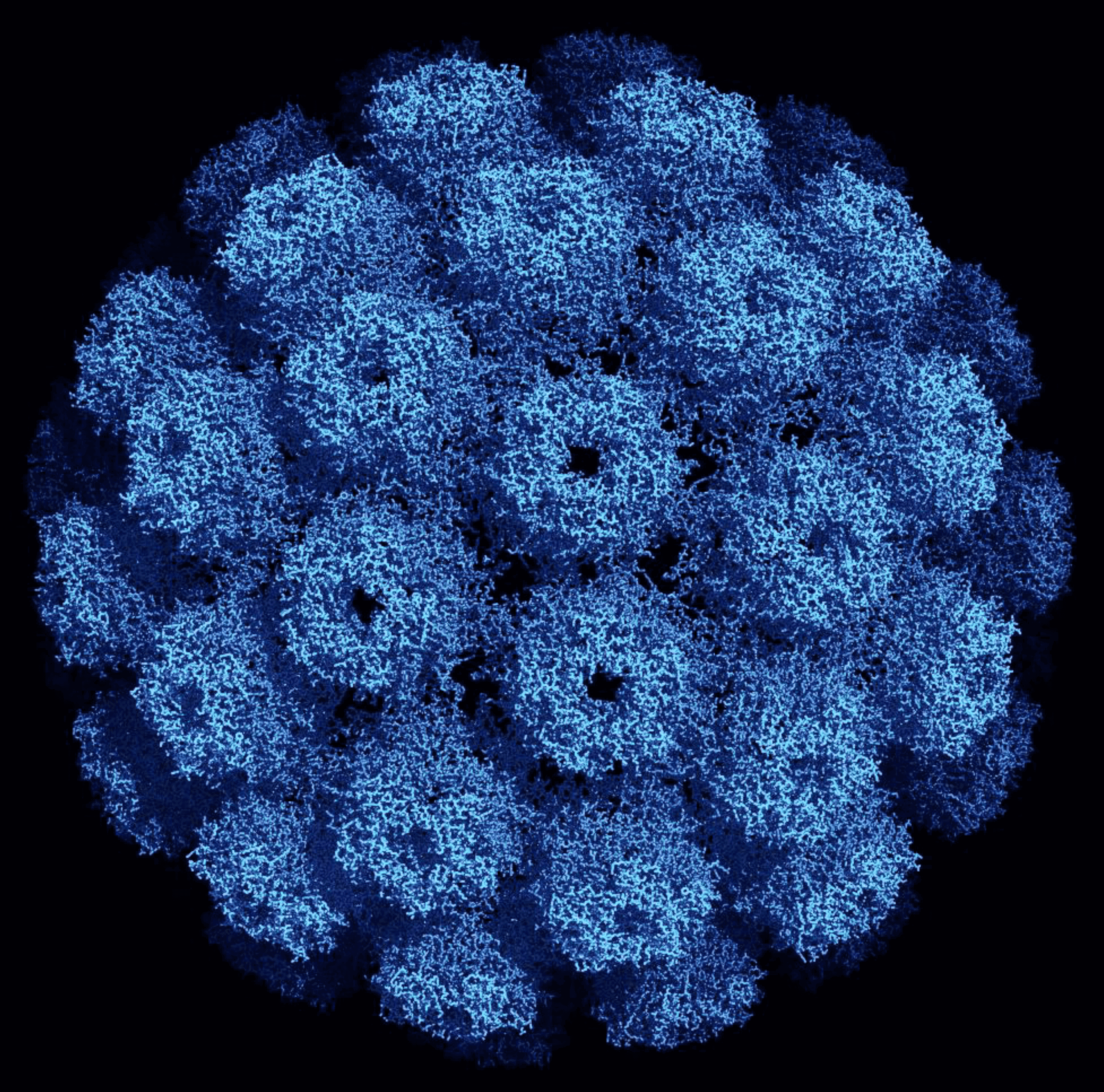 |
| Figure 1. . Polyomaviridae. Three dimensional structure of an SV40 particle at 3.1 Å resolution obtained using X-ray diffraction (Protein Data Base ID 1SVA, (Stehle et al., 1996)). The pentameric VP1 subunits are tied together by extended C-terminal arms. The diameter of this particle is about 500 Angstroms (Å) or 50 nm. Reproduced with permission obtained from RCSB Protein Data Bank. |
Physicochemical and physical properties
Virus particles have a sedimentation coefficient S20,w of 240S. Infectious particles have a buoyant density in CsCl of 1.34 g/cm3, and empty capsids have 1.29 g/cm3. Virions are stable at 50oC for 1 h, but are unstable under these conditions in the presence of 1M MgCl2. Being naked viruses, they are also resistant to lipid solvents (Imperiale and Major 2013). When VP1 is expressed alone, it can form virus-like particles (VLPs) ranging in size from 20 nm to 60 nm. Recombinant VP1 can also self-assemble and package dsDNA molecules of 5–9.4 kbp. However, VLPs seem to package a lower level of histones than native virions (Hurdiss et al., 2016, Fang et al., 2012).
Nucleic acid
The genome of most polyomaviruses is approximately 5,000 bp (Figure 2. Polyomaviridae). The genomes of members of the recognized species vary from 3,962 bp (giant guitar fish polyoma virus (GfPyV1; KP264963, species Rhynchobatus djiddensis polyomavirus 1) to 7,369 bp (black sea bass-associated polyomavirus 1 (BassPyV1; KP071318, species Centropristis striata polyomavirus 1). Of the known human polyomaviruses, Merkel cell polyomavirus (MCPyV; HM011556, species Human polyomavirus 5), has the largest genome (5,387 bp), and Saint Louis polyomavirus (STLPyV; JX463183, species Human polyomavirus 11), has the smallest (4,776 bp). Among known bird polyomaviruses, budgerigar fledgling disease virus (BFDV; AF241168, species Aves polyomavirus 1) has a genome of 4,981 bp, and the largest so far known avian polyomavirus genome is 5,421 bp (canary polyomavirus (CaPyV; GU345044, species Serinus canaria polyomavirus 1) (Calvignac-Spencer et al., 2016).
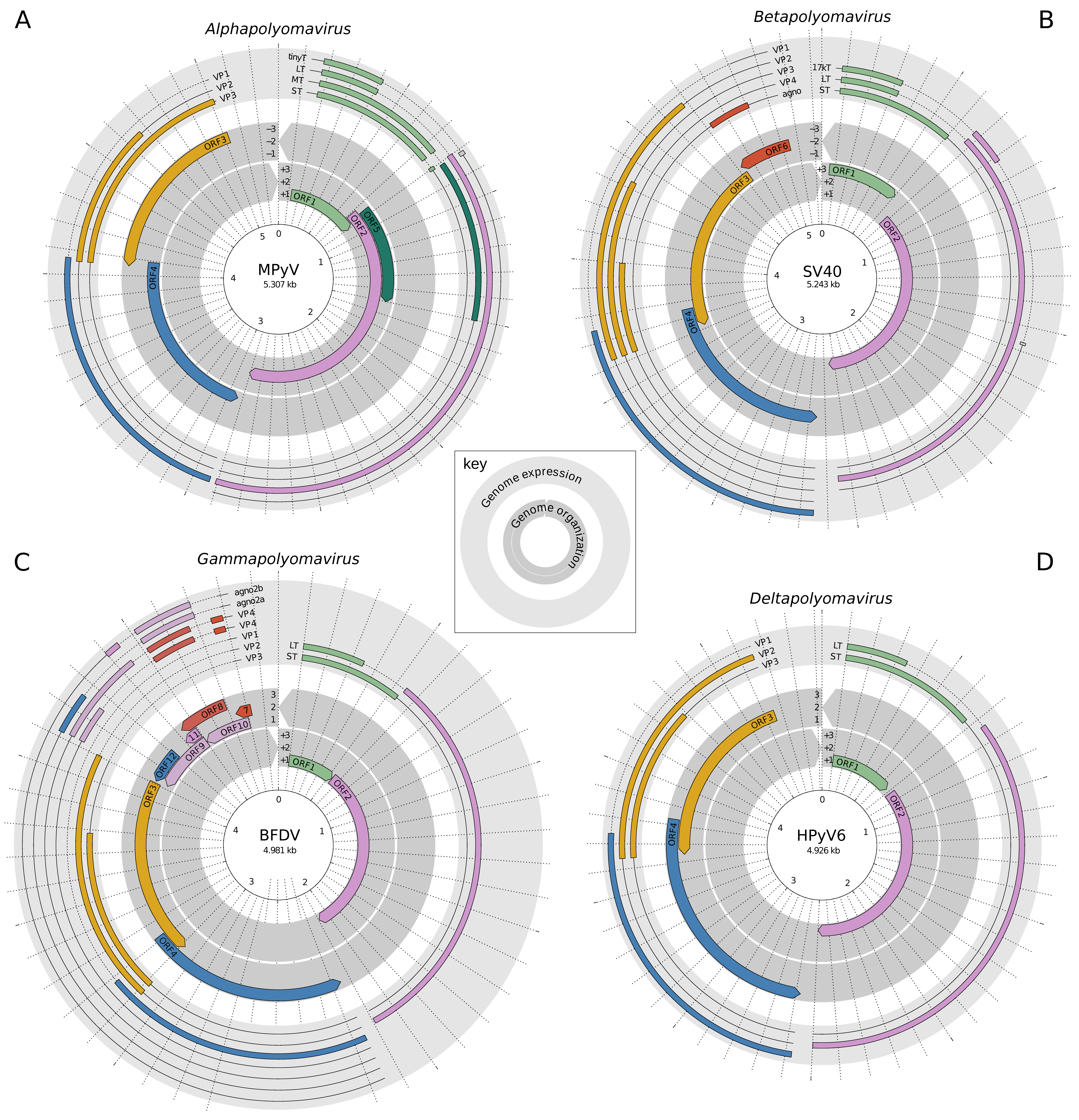 |
| Figure 2. Polyomaviridae. Genome organizations and expression products for members of four polyomavirus genera. Genomic annotations were taken from NCBI GenBank for the following viruses: (A) mouse polyomavirus (MPyV; AF442959, species Mus musculus polyomavirus 1, genus Alphapolyomavirus); (B) simian virus 40 (SV40; J02400, species Macaca mulatta polyomavirus, genus Betapolyomavirus); (C) budgerigar fledgling disease virus (BFDV; AF241168, species Aves polyomavirus 1, genus Gammapolyomavirus); (D) human polyomavirus 6 (HPyV6; HM011560, species Human polyomavirus 6, genus Deltapolyomavirus). Each panel has two circular layers, as detailed in the key inset at the center. The inner layer depicts the six reading frames of the genome, with frame +1 starting at the first nucleotide of the linearized genome clock-wise and frame -1 starting at the last nucleotide in reverse direction; open reading frames (ORFs) are indicated by colors. The outer layer depicts spliced transcripts, with coding exons colored according to the respective ORFs. Untranslated regions and introns are shown as solid and dashed lines, respectively. The radial axis is in bp units of 100. Genome organisations and expression products for members of the two other polyomavirus genera are very similar, the only significant exception being the presence of two introns in the LTAg coding sequence of members of the genus Epsilonpolymavirus (Ehlers et al., 2019). Figure layout adapted from (Kazem et al., 2016). |
Proteins
There are three capsid proteins, VP1, VP2 and VP3 (see below).
Lipids
Free lipids are not present in mature virions. However, covalent linkage of myristic acid to glycine at position 2 (Gly-2) in the VP2 proteins of mouse polyomavirus (MPyV; AF442959, species Mus musculus polyomavirus 1), and SV40 virions has been reported (Streuli and Griffin 1987). Gly-2 is conserved in VP2 of all known polyomaviruses, and its mutation in MPyV hampers viral replication and reduces infectivity and pathogenicity (Krauzewicz et al., 1990, Mannova et al., 2002, Sahli et al., 1993).
Carbohydrates
None present.
Genome organization and replication
Proteins
Typical polyomavirus genomes encode two regulatory proteins (large tumor antigen or LTAg and small tumor antigen or STAg) that are expressed early during infection, and three capsid proteins, VP1, VP2 and VP3, which are expressed after the onset of viral DNA replication and are therefore designated as late proteins. However, some polyomaviruses produce additional early and late proteins (Table 2. Polyomaviridae). A well-populated lineage of polyomaviruses that infect a diverse range of hosts, including humans, encode an additional open reading frame (ORF) that is expressed either as a separate protein (alternative tumor antigen or ALTO) or as the second exon of middle tumor antigen (MTAg) early during infection (Carter et al., 2013, Lauber et al., 2015, van der Meijden et al., 2015). An additional late protein encoded by simian virus 40 (SV40) is named VP4. However, this is a regulatory protein involved in egress and does not form a structural component of virus particles. Putative ORFs for VP4 are also present in BK polyomavirus (BKPyV; V01108, species Human polyomavirus 1), JC polyomavirus (JCPyV; J02226, species Human polyomavirus 2), and some non-human primate polyomaviruses (Ehlers and Moens 2014). SV40, BKPyV and JCPyV express a peptide, designated agnoprotein (Saribas et al., 2016). This regulatory protein plays a role in virus transcription, maturation and egress. A putative gene encoding a peptide with amino acid similarity to agnoprotein is present in genomes of other mammalian polyomaviruses (Ehlers and Moens 2014). Like SV40 VP4, agnoprotein is not a component of the virus particle. Avian polyomaviruses encode a unique fourth capsid protein designated VP4. This protein lacks similarity to SV40 VP4. It may also play a role in genome packaging and capsid formation (Shen et al., 2011).
Table 2A. Polyomaviridae. Human polyomavirus-encoded proteins. The proteins of SV40 are also included. The number of amino acid residues for each protein is given.
| Genus Betapolyomavirus | Genus Alphapolyomavirus | Genus Deltapolyomavirus | Genus Epsilonpolyomavirus | Genus Zetapolyomavirus | ||||||||||
| Protein | SV40 | JCPyV | KIPyV | WUPyV | MCPyV | TSPyV | NJPyV | HPyV6 | HPyV7 | MWPyV | STLPyV | BPyV | DPyV | |
| LTAga | 708 | 688 | 641 | 648 | 817 | 697 | 711 | 669 | 671 | 668 | 659 | 587 | 635 | |
| STAg | 172 | 172 | 191 | 194 | 186 | 198 | 183 | 190 | 193 | 199 | 195 | 125 | 170 | |
| MTAg | 332 | 229b | ||||||||||||
| ALTO | 248/250 | 131 | 299b | |||||||||||
| T’135 | 135 | |||||||||||||
| T’136 | 136 | |||||||||||||
| T’165 | 165 | |||||||||||||
| 57kT | 532 | |||||||||||||
| 21kT | 184 | |||||||||||||
| 17kT | 135 | |||||||||||||
| Tiny T | 85 | |||||||||||||
| VP1 | 364 | 354 | 378 | 369 | 423 | 376 | 489 | 387 | 380 | 403 | 401 | 366 | 359 | |
| VP2 | 352 | 344 | 400 | 415 | 241 | 313 | 232 | 336 | 329 | 310 | 303 | 354 | 209 | |
| VP3 | 234 | 225 | 257 | 272 | absent | 195 | 187 | 215 | 209 | 200 | 195 | 333 | 348 | |
| VP4 | 125 | 116b | ||||||||||||
| agno | 62 | 71 | 119 | |||||||||||
aAbbreviations: agno=agnoprotein; ALTO=alternative tumor antigen; MTAg=middle tumor antigen; LTAg=large tumor antigen; STAg=small tumor antigen. Virus name abbreviations are found in the Member species tables
bputative, existence not proven.
Table 2B. Polyomaviridae. Avian and fish polyomavirus-encoded proteins. The number of amino acid residues for each protein is given.
| Genus Gammapolyomavirus | currently not assigned to a genus | |||||||||||||
| Protein | GHPV | BFDV | CPyV | Butcherbird PyV | AdPyV | FPyV | CaPyV | EgouPyV1 | HunFPyV | BassPyV | GfPyV1 | SspPyV | SaurPyV1 | TberPyV1 |
| LTAg | 636 | 599 | 636 | 640 | 660 | 612 | 625 | 634 | 612 | 783 | 597 | 762 | 832 | 672 |
| STAg | 160 | 145 | 166 | 170 | 178 | 166 | 167 | 170 | 166 | 74 | ||||
| alTa | 717 | |||||||||||||
| ORF1 | 119 | |||||||||||||
| ORF2 | 111 | |||||||||||||
| VP1 | 353 | 343 | 353 | 357 | 360 | 358 | 356 | 362 | 358 | 353 | 277 | 324 | 361 | 323 |
| sVP1 | 437 | |||||||||||||
| VP2 | 326 | 341 | 333 | 331 | 349 | 354 | 369 | 339 | 335 | 599 | 242 | 556 | 645 | 509 |
| VP3 | 217 | 235 | 227 | 220 | 234 | 244 | 245 | 229 | 243 | |||||
| VP4 | 169 | 176 | 150 | 137 | 205 | |||||||||
| VP4D | 112 | |||||||||||||
| ORF-X | 432 | 205 | ||||||||||||
aAbbreviations: alT=alternative T; sVp1=N-terminal extended VP1. Virus name abbreviations are found in the Member species tables.
Genome Organisation
The genome contains two distinct transcriptional regions: the early and the late region, referring to the stage of productive infection during which they are transcribed. A non-coding control region (NCCR) encompassing the origin of DNA replication and the promoters and transcriptional enhancers for early and late region transcription separates the early and late region (Figure 2. Polyomaviridae). Transcription of the early region results in a single precursor mRNA from which different transcripts are generated through alternative splicing. The major translational products generated from these spliced mRNAs are the regulatory proteins LTAg and STAg. Several polyomaviruses express additional early proteins or their genomes encode additional putative early proteins (Table 2. Polyomaviridae). The late region is transcribed from the complementary strand and in opposite direction from the early region. The late region codes for at least two late proteins, VP1 and VP2, which are translated from different mRNAs as a result of alternative splicing (Figure 2. Polyomaviridae). For most polyomaviruses, a third structural protein (VP3) is generated from the same transcript as the VP2 protein by use of an internal, in-frame start codon (Imperiale and Major 2013). For SV40, a VP4 was identified in the same ORF as VP2/VP3, and was found to be necessary for lysis of infected cells. This VP4 ORF is conserved in BKPyV and JCPyV, but expression of this protein remains to be proven (Daniels et al., 2007). Some polyomaviruses have a short ORF upstream of the start codon of VP1, referred to as the agno gene, that encodes a hydrophobic protein known as agnoprotein. The VP4 protein of bird polyomaviruses is derived from an additional ORF located upstream of the VP2-encoding late mRNA (Johne and Muller 2007). Some mammalian polyomavirus lineages are the result of ancient recombination events between early and late regions of the genome (Lim et al., 2013, Tao et al., 2013).
Transcription and replication
Transcription of the early and late genes is directed by the NCCR, which is located between the early and late regions. The NCCR shows the highest sequence variability among polyomaviruses. However, conserved LTAg-binding motifs (5'-GRGGC-3') are present throughout the family. The early promoter has a TATA-box, whereas the late promoter lacks such a motif. The NCCR contains multiple binding sites for cellular transcription factors (Imperiale and Major 2013).
LTAg is involved in the switch from early to late viral gene expression. At low concentrations of LTAg, this protein will occupy high affinity LTAg-binding motifs and stimulate early transcription and viral DNA replication. Later in infection, LTAg will also bind to low affinity binding motifs as LTAg concentration increases (Imperiale and Major 2013). These motifs are located downstream from the TATA box. As a result, LTAg prevents early transcription by blocking passage of the RNA polymerase II complex. LTAg is also involved in the switch to late transcription and facilitates late transcription by recruiting transcription factors. For SV40, it was shown that a cellular repressor present in low concentration prevents late transcription (Wiley et al., 1993). As the viral genome replicates, more viral genome copies are produced and the repressor is competed out, allowing late transcription. Viral DNA that is transcribed during the late phase of infection seems to be nucleosome-free in the NCCR, allowing more active transcription (Imperiale and Major 2013).
The NCCRs of clinical isolates of BKPyV and JCPyV show extensive rearrangements. This hypervariability affects viral transcription, replication and cytopathology in cell cultures (Gosert et al., 2008, Gosert et al., 2010). The NCCRs of other polyomaviruses have been less studied, but so far show only minor nucleotide changes or short deletions. For example, KI polyomavirus (KIPyV; EF127960, species Human polyomavirus 3), MCPyV, Trichodysplasia spinulosa-associated polyomavirus (TSPyV; GU989205, species Human polyomavirus 8) and human polyomavirus 9 (HPyV9; HQ696595, species Human polyomavirus 9) strains with mutations in the NCCR have been described (Kazem et al., 2016, Lednicky et al., 2014, Schowalter et al., 2010, Song et al., 2016), but the biological consequences of these mutations have not been elucidated.
The best-studied polyomaviruses (SV40, BKPyV, JCPyV, MCPyV and MPyV) all encode a microRNA (miRNA) in the late genome region (DeCaprio and Garcea 2013, Sullivan et al., 2009). This miRNA is complementary to LTAg mRNA and thus can downregulate LTAg expression. SV40 miRNA reduces cytokine production and the susceptibility of virus-infected cells to cytotoxic T cells. Moreover, SV40 miRNA can interfere with the JNK and p38 mitogen-activated protein kinase pathway by targeting dual-specificity protein phosphatase DUSP8, although the biological consequence of this interaction is not known (Chen et al., 2013). BKPyV and JCPyV miRNAs target ULBP3, which is the ligand of the natural killer group 2 member D (NKG2D). This may prevent the elimination of BKPyV- and JCPyV-infected cells by natural killer cells (Bauman et al., 2011). MCPyV miRNA may downregulate expression of the cellular proteins RUNX1 (a transcription factor), RBM9/FOX2 (a splicing factor), and MECP2 (methyl-CpG binding protein 2, a protein involved in gene regulation) (Lee et al., 2011), but the implication for MCPyV biology remains elusive. Raccoon polyomavirus (RacPyV; JQ178241, species Procyon lotor polyomavirus 1), Gorilla gorilla gorilla polyomavirus 1 (GgorgPyV1; HQ385752, species Gorilla gorilla polyomavirus 1) and Pan troglodytes verus polyomavirus 2a (PtrovPyV2a; HQ385748, species Pan troglodytes polyomavirus 3) are so far the only non-human polyomaviruses shown to encode bona fide miRNAs (Chen et al., 2015), and putative miRNAs are predicted at similar locations in the genomes of other polyomaviruses, including Cercopithecus erythrotis polyomavirus 1 (CeryPyV1; JX159985, species Cercopithecus erythrotis polyomavirus 1), vervet monkey polyomavirus 2 (VmPyV2; AB767299, species Chlorocebus pygerythrus polyomavirus 2) and yellow baboon polyomavirus 2 (YbPyV2; AB767295, species Papio cynocephalus polyomavirus 2) (Ehlers and Moens 2014).
Biology
Host range and evolution
Polyomaviruses infect members of many mammalian and avian orders and were long considered as parasites of tetrapods. However, complete polyomavirus genome sequences have now been determined from members of at least five fish species: black sea bass (Centropristis striata), giant guitarfish (Rhynchobatus djiddensis), gilt-head bream (Sparus aurata), sharp-spined notothen (Trematomus pennellii) and emerald notothen (Trematomus bernacchii). The viruses BassPyV1, GfPyV1, Sparus aurata polyomavirus 1 (SaurPyV1; KX643371), sharp-spined notothenia polyomavirus (SspPyV; KP768176) and emerald notothen polyomavirus 1 (TberPyV1; MG800627) have now been assigned to the species Centropristis striata polyomavirus 1, Rhynchobatus djiddensis polyomavirus 1, Sparus aurata polyomavirus 1, Trematomus pennellii polyomavirus 1 and Trematomus bernacchii polyomavirus 1, respectively (Calvignac-Spencer et al., 2016). Polyomavirus-like sequences have also been recovered from arthropods by mining whole genome sequences, transcriptome shotgun assemblies and short read archives (Buck et al., 2016). These sequences did not allow for the identification of bona fide polyomaviruses as they were (i) partial and integrated in the genome of their hosts (spiders and bristletails), or (ii) complete episomal sequences that did not exhibit the typical genome organization of polyomaviruses (polyomavirus associated with Baja California bark scorpion; Centruroides exilicauda). These sequences may indicate that polyomaviruses also infect or previously infected invertebrates and testify of an ancient association of polyomaviruses or polyomavirus-like entities with animals (Buck et al., 2016).
Polyomavirus diversity has been shaped by a complex mixture of evolutionary processes (Buck et al., 2016, Madinda et al., 2016). Polyomaviruses are generally host-specific and their diversification has thus likely been strongly influenced by divergence with their hosts. Calibrating such co-divergence events with molecular clock analyses suggests relatively slow rates of molecular evolution (ca. 10-8 substitutions per site per year; (Buck et al., 2016, Madinda et al., 2016, Krumbholz et al., 2009)). Lineage duplications have also occurred repeatedly, as exemplified by the 13 human and 8 chimpanzee polyomaviruses identified to date. Finally, recombination has also played a role in polyomavirus evolution. Recombination has reshuffled the early and late regions of long-diverged polyomavirus genomes, e.g. KIPyV, WU polyomavirus (WUPyV; EF444549, species Human polyomavirus 4), human polyomavirus 6 (HPyV6; HM011560, species Human polyomavirus 6), human polyomavirus 7 (HPyV7; HM011566, species Human polyomavirus 7), and STLPyV (Lim et al., 2013, Tao et al., 2013, Buck et al., 2016), as well as fragments of polyomaviruses and viruses belonging to other known virus families and or unknown groups, as exemplified by the discoveries of the bandicoot papillomatosis carcinomatosis virus types 1 and 2 and the virus causing viral endothelial cell necrosis of eel (Bennett et al., 2008, Mizutani et al., 2011, Woolford et al., 2008).
Epidemiology and biological properties of mammalian polyomaviruses
The exact routes of infection and transmission, and the identities of genuine host cells, are unclear for most mammalian polyomaviruses. The skin seems to be a natural habitat for MCPyV, HPyV6, HPyV7 and TSPyV, with direct skin-to-skin contact as a source of transmission. DNA from MW polyomavirus (MWPyV; JQ898291, species Human polyomavirus 10), and STL polyomavirus (STLPyV; JX463183, species Human polyomavirus 11) has been detected in the gastrointestinal tract, and the shedding of other polyomaviruses in urine and faeces. This suggests faecal/urine-oral route of transmission (Schowalter et al., 2010, Liu et al., 2016). Respiratory transmission is also possible because JCPyV, BKPyV, KIPyV and WUPyV are often found in tonsillar tissue and respiratory aspirates, suggesting an aerogenic route of infection. Sewage water has also been found to contain low level of polyomaviruses, indicating that virus infection may be acquired through drinking and eating (Bofill-Mas et al., 2013). Vertical transmission has been suggested for BKPyV and JCPyV, but convincing proof is lacking.
Serological studies have shown that human polyomaviruses are ubiquitous in healthy individuals, with seropositivity varying from 40% to 90%, depending on the virus (Kean et al., 2009, Gossai et al., 2016). Primary infection occurs typically during early childhood, and sustained antibody titers throughout life indicate a persistent infection. There is a tendency towards higher seropositivity in older people.
The cells that host human polyomaviruses in vivo, are poorly characterized. BKPyV persists in renal proximal tubular epithelial cells, and salivary gland cells may also be permissive. JCPyV is latent in the kidney, but can reactivate under immunocompromised conditions. JCPyV infects glial cells, and MCPyV seems to infect dermal fibroblasts (DeCaprio and Garcea 2013, Liu et al., 2016). TSPyV infects the inner root sheath cells of the hair follicle (Kazem et al., 2012). The DNA of the other human polyomaviruses has been detected in various cell types, but whether they represent bona fide host cells remains to be determined.
MPyV is the most well studied PyV with regards to infection in vivo. MPyV can potentially infect a very broad range of cells, with more than 30 different cell types infected after inoculation of MPyV in newborn mice (Dawe et al., 1987). After intranasal or subcutaneous injection of MPyV in newborn mice, replication has been observed in a wide range of organs, including liver, spleen, kidneys, lungs and bones (Demengeot et al., 1990, Dubensky et al., 1984). After inoculation of adult immunocompetent mice, MPyV replication is more limited, peaking at 1-2 weeks post infection and being mainly restricted to bone, heart and lymph nodes (Berke and Dalianis 1993), although other organs are also involved. In adult immunocompetent mice, MPyV infection is cleared within two months (Berke and Dalianis 1993), whereas in newborn mice infection persists longer, especially in the kidneys (Berke et al., 1996). In immunodeficient mice, MPyV infection is mostly systemic and, depending on the immunodeficiency, may be lethal (Berke et al., 1998).
Several mammalian polyomaviruses require cellular glycans as host cell receptors. Gangliosides GD1b and GT1b are required for the attachment and entry of BKPyV into human renal proximal tubular epithelial cells, and GD2 and GD3 increase the infection efficiency (O'Hara et al., 2014). JCPyV uses GT1b, GD1b and GD2. Unique for JCPyV is that it also makes contact with the pentasaccharide lactoseries tetrasaccharide c, and serotonin receptor 5HT2A acts as a possible co-receptor. MCPyV binds GT1b and uses glycosaminoglycans as possible co-receptors. HPyV9 VP1 preferentially binds sialyllactosamine compounds terminating in 5-N-glycolyl neuraminic acid over those terminating in 5-N-acetyl neuraminic acid. SV40 utilizes GM1 as a receptor, and MHC class I functions as a possible co-receptor. GD1a and GT1b are receptors identified for MPyV, and a4b1-integrin can function as a co-receptor. HPyV6 and HPyV7 may employ non-ganglioside receptorsas suggested by high-resolution X-ray studies of the VP1 proteins. This was confirmed by the absence of interaction between VP1 and a2,3- and a2,6-linked sialylated glycans in solution, by using nuclear magnetic resonance spectroscopy and flow-cytometric single cell-binding studies (Stroh et al., 2014). Apart from receptor variation, the site on VP1 that is used for interaction varies among mammalian polyomaviruses (Stroh et al., 2015).
After entering the cells, virion decapsidation is initiated in the cytosol. Partial uncoating exposes the nuclear localization signals in VP2/VP3 and helps to guide the particles into the nucleus, where further disassembly occurs. The viral genome remains episomal. At an early stage in infection, transcription occurs from one strand and in one direction, giving rise to mRNAs encoding LTAg, STAg and alternative early proteins. LTAg autoregulates its own transcription and is the only viral protein required for replication. The DnaJ domain in the N-terminal part of LTAg as well as the ATPase/helicase activity, are necessary for efficient viral DNA replication. LTAg of most polyomaviruses binds repeats of the 5'-GRGGC-3' motif, while LTAg of Gammapolyomavirus interacts with the palindromic motif 5'-CC(W)6GG-3'. LTAg binds the tumour suppressor proteins p53 and the retinoblastoma family members, driving the cell into the S phase. STAg also contains the DnaJ domain and can bind to and inactivate cellular protein phosphatase 2A (PP2A), which leads to activation of cyclin D1 and cyclin A, promoting G1 to S phase progression. The middle T antigen expressed contains the DnaJ domain and binds PP2A. The role of the alternative early proteins is less well understood, although it has been suggested that MTAg/ALTO is involved in adaptation of polyomavirus species from many different hosts, including bats, rodents, monkeys, and hominids including humans (Lauber et al., 2015). Some of the alternative early proteins can bind the retinoblastoma family members. MCPyV ALTO protein is not required for replication.
After viral replication has been initiated, the late genes are transcribed from the opposite strand in an opposite direction from the early genes. The capsid proteins are synthesized in the cytoplasm and transported into the nucleus, where assembly with the viral genome into viral particles occurs. The viral genome is packed with histones H2A, H2B, H3 and H4. Viruses are released by lysis-dependent or -independent mechanisms (Imperiale and Major 2013, DeCaprio and Garcea 2013). The SV40 VP4 protein and the JCPyV agnoprotein have been shown to facilitate viral egress. Agnoprotein is also involved in virus assembly, as BKPyV, JCPyV and SV40 mutants lacking the agnoprotein display impaired production of infectious virus particles (Saribas et al., 2016). For MCPyV, it has been suggested that dermal fibroblasts are the genuine host cells where infectious virus particles are produced. Infection of non-permissive Merkel cells that do not sustain the viral life cycle results in integration of the MCPyV genome and transformation (Liu et al., 2016).
Epidemiology and biological properties of avian polyomaviruses
Information on avian polyomavirus infection, route of transmission, spread and seropositivity is scarce. BFDV seems to be common in many, but not all, captive and wild psittaccine bird species tested thus far (Deb et al., 2010, Herrera et al., 2001, Khan et al., 2000, Phalen et al., 1993, Raidal et al., 1998, Wainright et al., 1987). Birds belonging to other orders have also been shown to be infected with polyomaviruses. In some cases, these infections appear to be common. For example, antibodies to goose haemorrhagic polyomavirus (GHPV; AY140894, species Anser anser polyomavirus 1) were detected in sera of 43% of healthy geese (Zielonka et al., 2006). The presence of polyomaviruses in apparently healthy birds suggests asymptomatic or subclinical symptoms. Virus is shed in droppings and feather dander, and is transmitted by direct contact, contaminated aerosols or infected fomites. Little is known on how avian polyomaviruses spread after initial infection. In parrots exposed to BFDV, virus could be detected in blood, suggesting that the virus can spread by viremia (Phalen et al., 2000).
Epidemiology and biological properties of non-tetrapod polyomaviruses
Five polyomaviruses isolated from fish, BassPyV1, GfPyV1, SspPyV, SaurPyV1 and TberPyV1, have been recognized as members of different species; these have not yet been assigned to a genus. The BassPyV1 genome was determined by deep sequencing of a mixed tissue sample including scales, skin, lateral muscle, mouth, eye, anal/genital opening, swim bladder and liver. Nothing is known about BassPyV1 biology.
Polyomavirus-related sequences from a scorpion have been reported (see above) but are currently not classified (Calvignac-Spencer et al., 2016).
Pathogenicity
Human polyomavirus infections in healthy individuals have not been associated with clinical symptoms or diseases. Immunocompromising conditions can lead to human polyomavirus reactivation and clinical diseases. BKPyV can cause nephropathy in renal transplant patients and is linked to hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation. BKPyV has been suggested to play a role in prostate cancer. JCPyV is associated with progressive multifocal leukoencephalopathy in immunocompromised patients, especially HIV/AIDS-positive individuals, and may be involved in colon and brain cancer. MCPyV is linked to Merkel cell carcinoma, a rare but aggressive skin cancer (Feng et al., 2008). TSPyV is observed in trichodysplasia spinulosa, a rare skin disease exclusively seen in immunocompromised patients (van der Meijden et al., 2010). HPyV7 DNA and LTAg expression were detected in thymic epithelial tumours. However, the role of HPyV7 and other human polyomaviruses (except MCPyV) in cancer is disputed (DeCaprio and Garcea 2013, Toptan et al., 2016).
Despite their ability to transform cells in culture and induce tumours in animal models, other mammalian polyomaviruses do not seem to be involved in cancer in their natural host. One known exception is RacPyV which may contribute to the development of malignant brain tumours in raccoons (Dela Cruz et al., 2013). The poliovirus vaccination program (1955-1961) with SV40-contaminated vaccine has unintendedly introduced SV40 into the human population. SV40 alone or together with exposure to asbestos has been linked to brain tumours and mesotheliomaespectively, but the evidence is insufficient to confirm SV40 as a cause of these cancers (Qi et al., 2011).
Avian polyomaviruses cause haemorrhage, lethargy and death in birds, but none of the known avian polyomaviruses induces tumours in animal models or their natural host. BFDV was able to transform hamster embryo cells, but not murine 3T3 cells in vitro (Dykstra et al., 1984, Lehn and Muller 1986).
Antigenicity
The polyomavirus major capsid protein VP1 determines antigenicity. VP1 shows extended and structurally variable surface loops that emanate from a conserved b-sheet core structure. These surface loops are referred to as the BC-, DE-, EF-, GH and HI-loops (Stroh et al., 2014). The surface-exposed BC-loop is highly antigenic, and it is markedly divergent in the VP1 proteins of polyomaviruses, which may explain why little or no serological cross-reactivity is observed. Enzyme immunoassays (EIAs) with recombinant VP1, VP1 pentamers or VP1-based VLPs are now commonly used to distinguish antibodies against members of polyomavirus species. These assays can also be used to discriminate among isolates of a particular polyomavirus species, as has been shown for BKPyV and MCPyV antigenic variants that are sometimes designated serotypes (Carter et al., 2009, Kean et al., 2009, Pastrana et al., 2012). Antigenicity and seroprevalence in nonhuman mammals have been poorly investigated. Sera from raccoons with and without tumours were tested for antibodies against RacPyV VP1 protein by using a VLP-based ELISA. Juvenile raccoons had a seropositivity of 16.8%, whereas 45.7% of samples from adult raccoons were seropositive. Antibodies were significantly more common in raccoons with tumours than in healthy animals (Church et al., 2016). Antibodies specific for chimpanzee polyomaviruses were detected in captive and wild-caught animals by using a VLP-based ELISA, and sera from captive and free-ranging macaque species contained antibodies that cross-reacted in a SV40-based ELISA (Verschoor et al., 2008, Zielonka et al., 2011). Another study showed that polyomavirus VP1 antibodies are very common in nonhuman primates (Scuda et al., 2013).
Derivation of names
Polyomaviridae: from Greek poly, meaning “many”, and oma, denoting “tumours”
Alphapolyomavirus, Betapolyomavirus, Gammapolyomavirus, Deltapolyomavirus, Epsilonpolyomavirus and Zetapolyomavirus: first 6 letters in the Greek alphabet
Genus demarcation criteria
Reconstructed evolutionary relationships are used for the delineation of genera and derived from analyses of LTAg amino acid sequences (Calvignac-Spencer et al., 2016). LTAg is used instead of whole genome analyses, in order to avoid the confounding effect of recombination that has occurred between early and late regions of the genome in some mammalian lineages. Six genera are delineated (Alphapolyomavirus, Betapolyomavirus, Gammapolyomavirus and Deltapolyomavirus), and 112 species have been assigned to these genera (Calvignac-Spencer et al., 2016). Another five species are unassigned in the family.
Relationships within the family
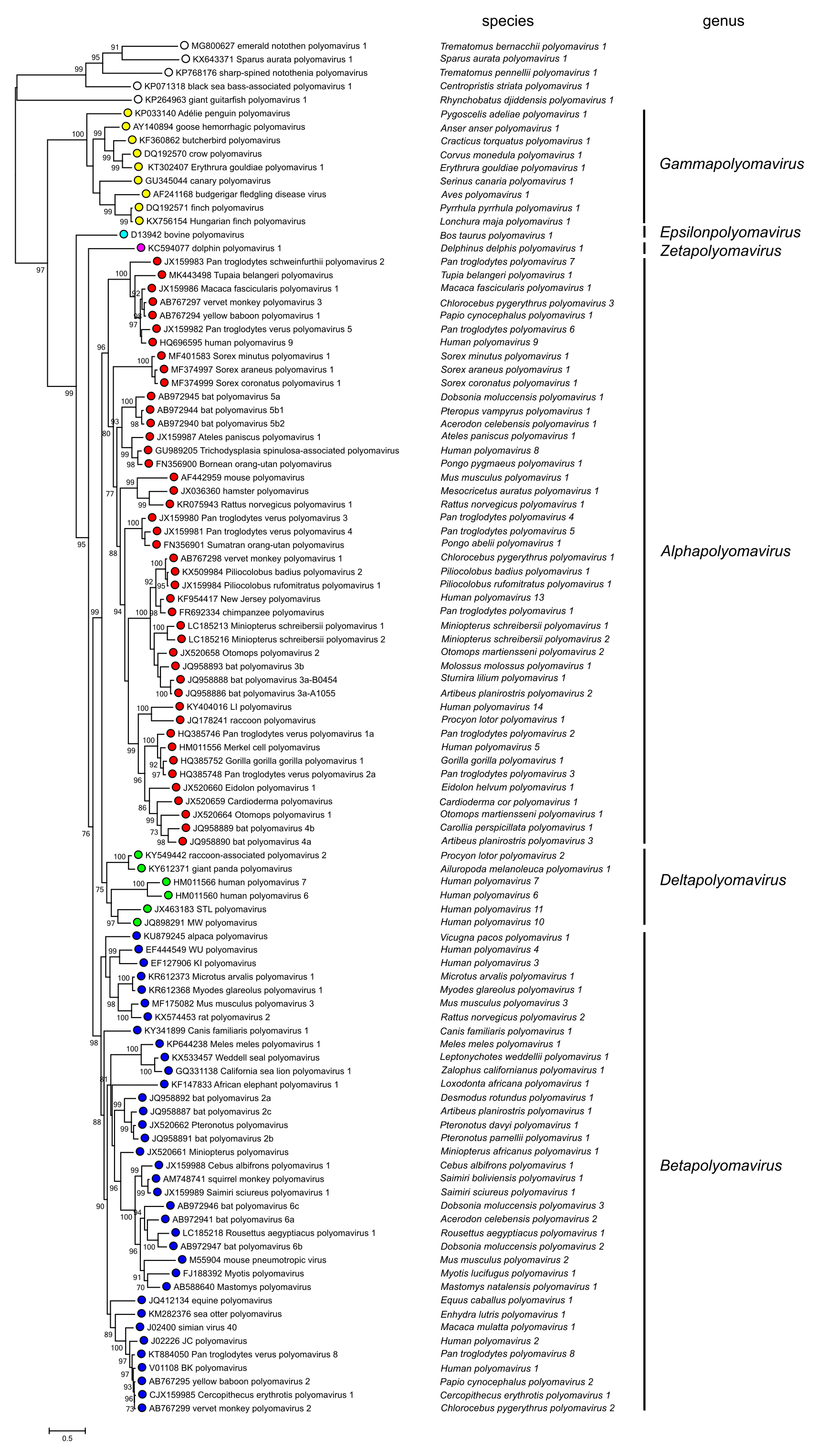
Phylogenetic relationships among polyomaviruses based on the amino acid sequence of LTAg are shown in Figure 3. Polyomaviridae.
|
| Figure 3. Polyomaviridae. Phylogenetic relationships of polyomaviruses based on conserved amino acid blocks of the LTAg coding sequence. Polyomaviruses are denoted by Genbank accession number, virus name, and species name; viruses are grouped into genera by both colouring and labels at the right-hand side of the figure. Open circles indicate viruses in species unassigned to a genus, or unclassified viruses. For phylogenetic analyses, the previously published recommendations were followed (Calvignac-Spencer et al., 2016). Bayesian Monte Carlo Markov chain analyses generated a maximum clade credibility tree whose topology was essentially similar to the topology of the maximum likelihood tree presented in this figure. Bootstrap support is indicated where this was > 70%. This phylogenetic tree and corresponding sequence alignment are available to download from the Resources page. |
Based on evolutionary relationships, the Polyomaviridae Study Group has divided the family into six genera. Fish polyomaviruses may merit the establishment of further genera based on their clear phylogenetic distinctness from tetrapod polyomaviruses.
Relationships with other taxa
Bandicoot papillomatosis carcinomatosis virus types 1 and 2 (BPCV1 and BPCV2, respectively) have circular dsDNA genomes that are similar to those of members of the family Papillomaviridae in size (ca. 7.3–7.5 kbp) and possibly in some aspects of gene content, in that they encode putative papillomavirus L1 and L2 structural proteins. However, they also encode putative polyomavirus LTAgs and STAgs. The origin of these viruses is not clear, although it could be explained as being due to recombination between a polyomavirus and a papillomavirus. A virus causing endothelial cell necrosis in Japanese eels has been identified that has a circular dsDNA genome of 15,131 bp, and a related virus with a circular dsDNA genome of 14,334 bp has been discovered in diseased marbled eels. Both genomes contain a sequence that encodes an LTAg-like protein. However, other coding sequences do not display similarity to those of polyomaviruses, and the evolutionary history of these fish viruses is unclear.
Related, unclassified viruses
| Virus name | Accession number | Virus abbreviation |
| African green monkey polyomavirus (lymphotropic polyomavirus) | K02562 | LPV |
| rabbit kidney vacuolating virus | AB435232 | |
| simian virus 12 | AY614708 | SA12 |
| human polyomavirus 12 | JX308829 | HPyV12 |
Virus names and virus abbreviations are not official ICTV designations.
These viruses were previously assigned as separate species (Johne et al., 2011), but have been removed from the family as they currently do not meet the species criteria for various reasons (Calvignac-Spencer et al., 2016, Gedvilaite et al., 2017) . A number of polyomaviruses with full genome sequences from mammals (nonhuman primates, bats, even-toed ungulates, rodents and others), birds and fish are currently proposed as species or are not assigned as species, either because the data have not been published in scientific journals or because their hosts have not been determined unequivocally (see species definition criterion C1 and C3 in: (Calvignac-Spencer et al., 2016)). In addition, a plethora of subgenomic polyomavirus sequences from various hosts have been published. As full genomes were not determined (species definition criterion C1 (Calvignac-Spencer et al., 2016)), the corresponding viruses have not been assigned to species.