Family: Asfarviridae
Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release
Since only one genus is currently recognized, the family description corresponds to the genus description.
Genus Asfivirus
Type species African swine fever virus
Virion properties
Morphology
African swine fever virus (ASFV) virions consist of a nucleoprotein core structure, 70–100 nm in diameter, surrounded by an internal lipid layer and an icosahedral capsid, 170–190 nm in diameter, and an external lipid-containing envelope. The capsid exhibits icosahedral symmetry (T=189–217) corresponding to 1892–2172 capsomers (each capsomer is 13 nm in diameter and appears as a hexagonal prism with a central hole; intercapsomeric distance is 7.4–8.1 nm). Extracellular enveloped virions have a diameter of 175–215 nm (Figure 1).
Physicochemical and physical properties
Virion buoyant density is 1.095 g cm−3 in Percoll, 1.19–1.24 g cm−3 in CsCl; S20,W is about 3500S. Virions are sensitive to ether, chloroform and deoxycholate, and are inactivated at 60 °C within 30 min, but survive for years at 20 °C or 4 °C. Infectivity is stable over a wide pH range. Some infectious virus may survive treatment at pH4 or pH13. Infectivity is destroyed by some disinfectants (1% formaldehyde in 6 d, 2% NaOH in 1 d); paraphenylphenolic disinfectants are very effective. Virus is sensitive to irradiation.
Nucleic acid
The genome consists of a single molecule of linear, covalently close-ended, dsDNA 170–190 kbp in size (varying among isolates). The end sequences are present as two flip-flop forms that are inverted and complementary with respect to each other, and adjacent to both termini are identical tandem repeat arrays about 2.1 kbp long. The complete nucleotide sequences of 11 isolates have been determined. These include the tissue culture-adapted Ba71V isolate (ASFV-Ba71V) and 10 field isolates from Europe and Africa.
Proteins
Virions contain more than 50 proteins, including a number of enzymes and factors needed for early mRNA transcription and processing (Table 1). Enzymes packaged into virions include the multi-subunit RNA polymerase, polyA polymerase, guanylyl transferase and protein kinase. The inhibitor of apoptosis (IAP) homolog protein is also packaged in virions. Virion structural proteins characterized include on the outer envelope the CD2v protein (EP402R), p22 (pKP177R), p12 (pO61R), on the capsid shell the major capsid protein p72 (pB646L), p49 (pB438L), in the internal envelope p17 (pD117L), p54 or j13L (pE183L) and probably j18L (pE199L), j5R (pH108R). The products of a 220 kDa protein (pCP2475L) that is cleaved to give four structural proteins (p150, p37, p14 and p34), and the products of a 62 kDa protein (pCP530R) that is cleaved to give two structural proteins (p35 and p15) are localized in the matrix or inner core shell. A virus-encoded protease related to the SUMO-1-specific protease family is involved in cleavage of these polyproteins. Two DNA binding proteins, p10 (pA78R) and p14.5 (pE120R), are present in virions. The virus encodes components of a redox pathway, including the pB119L (or 9GL), pE248R and pA151R proteins, of which the pA151R and pB119L proteins are non-structural. The pB602L protein is a chaperone required for assembly of the p72 capsid protein into virions.
Other predicted proteins encoded by the virus include enzymes involved in nucleotide metabolism (ribonucleotide reductase, thymidine kinase, thymidylate kinase and deoxyuridine triphosphatase), DNA replication and repair or transcription (DNA polymerase, DNA polymerase X, DNA ligase, topoisomerase II, guanylyl transferase, three members of DNA helicase superfamily II, and AP endonuclease). Deletion of the thymidine kinase, AP endonuclease and deoxyuridine triphosphatase genes does not affect virus replication in tissue culture cell lines but reduces virus replication in fully differentiated non-dividing macrophages and reduces virulence of the virus in pigs. Two enzymes involved in post-translational protein modification (a ubiquitin-conjugating enzyme and a serine/threonine protein kinase) and an enzyme involved in synthesis of isoprenoid compounds (trans-prenyltransferase) are encoded by the virus.
Five different multigene families (MGF 110, MGF 360, MGF 530/505, MGF 300 and MGF 100) are found in genome regions close to the termini. Large length variations between genomes of different isolates are due to gain or loss of members of these multigene families. MGF 110 contains 14 members and individual isolates contain between 5 and 11 of these. MGF 360 has 22 members and between 11 and 18 copies are encoded by different isolates. MGF 530/505 has 11 members and between 8 and 10 copies are present. MGF 300 has 4 copies with 3 or 4 present and MGF 100 has 3 copies with 2 or 3 present. Members of families MGF 360 and 530 have been implicated as macrophage host range determinants, and deletion of 6 members of MGF 360 and 2 of MGF 530 results in an increase in type I interferon production. These 6 copies of MGF 360 and 1 or 2 copies of MGF 530 are deleted from the genome of an attenuated field isolate OURT88/3 and the tissue-culture adapted BA71V isolate.
Virus-encoded proteins that modulate the host response to virus infection include homologs of the apoptosis inhibitors Bcl2 and IAP. Both of these proteins inhibit apoptosis; the IAP homolog inhibits caspase 3 activity. A C-type lectin (pEP153R) has also been reported to inhibit apoptosis. Although the virus encodes proteins that inhibit apoptosis, apoptotic cells are observed at late stages of infection. The A238L protein inhibits transcriptional activation dependent on a number of different host transcription factors, including NFAT, NFkB and cJun, by inhibiting their transactivation mediated by p300. This protein also binds to and inhibits the host calcineurin phosphatase and is therefore predicted to inhibit calcineurin-dependent pathways. The A238L protein may therefore inhibit transcriptional activation in infected macrophages of a wide range of host immunomodulatory genes that are dependent on these factors. A virus protein, EP402R, which is similar to the host T cell adhesion protein CD2, is required for the hemadsorption of red blood cells around virus-infected cells and is also thought to mediate the adhesion of extracelluar virions to red blood cells. Deletion of the EP402R gene reduces virus dissemination in infected pigs and in vitro abrogates the ability of ASFV-infected cells to inhibit proliferation of bystander lymphocytes in response to mitogens. One protein (designated pNL-S, pl14L or pDP71L) is similar over a conserved C-terminal domain to a herpes simplex virus-encoded neurovirulence factor (ICP34.5) and host protein GADD34. These proteins all act to recruit cellular protein phosphatase 1 to dephosphorylate translation initiation factor eIF2α and inhibit global shut-off of translation. GADD34 and ICP34.5 are larger and have other demonstrated roles. One of the major ASFV-induced proteins, encoded by gene CP204L, interacts with the heterogeneous nuclear ribonucleoprotein K (hnRNP-K) with potential implications in the downregulation of host cell mRNA translation.
Table 1 Functions of African swine fever virus (ASFV) encoded proteins
| Gene function | Gene name | Predicted protein size (kDa) |
| Nucleotide metabolism, transcription, replication and repair |
|
|
| Thymidylate kinase | A240L | 27.8 |
| Thymidine kinase | K196R | 22.4 |
| dUTPase* | E165R | 18.3 |
| Ribonucleotide reductase (small subunit) | F334L | 39.8 |
| Ribonucleotide reductase (large subunit) | F778R | 87.5 |
| DNA polymerase α-like | G1211R | 139.8 |
| DNA topoisomerase type II* | P1192R | 135.5 |
| Proliferating cell nuclear antigen (PCNA)-like | E301R | 35.3 |
| DNA polymerase X-like* | O174L | 20.3 |
| DNA ligase* | NP419L | 48.2 |
| AP endonuclease class II* | E296R | 33.5 |
| RNA polymerase subunit 2 | EP1242L | 139.9 |
| RNA polymerase subunit 6 | C147L | 16.7 |
| RNA polymerase subunit 1 | NP1450L | 163.7 |
| RNA polymerase subunit 3 | H359L | 41.3 |
| RNA polymerase subunit 5 | D205R | 23.7 |
| RNA polymerase subunit 10 | CP80R |
|
| TFIIB like | C315R |
|
| Helicase superfamily II | A859L | 27.8 |
| Helicase superfamily II | F1055L | 123.9 |
| Helicase superfamily II | B962L | 109.6 |
| Helicase superfamily II | D1133L | 129.3 |
| Helicase superfamily II | Q706L | 80.4 |
| Helicase superfamily II | QP509L | 58.1 |
| Transcription factor SII | I243L | 28.6 |
| Guanylyl transferase* | NP868R | 29.9 |
| PolyA polymerase large subunit | C475L | 54.8 |
| FTS J-like methyl transferase domain | EP424R | 49.3 |
| ERCC4 nuclease domain | EP364R | 40.9 |
| Lambda-like exonuclease | D345L | 39.4 |
| VV A2L-like transcription factor | B385R | 45.3 |
| VV A7L-like transcription factor | G1340L | 155.0 |
| VV VLTF2-like late transcription factor | B175L | 20.3 |
| FCS-like finger DNA primase | C962R | 111.3 |
| Other enzymes |
|
|
| Prenyltransferase* | B318L | 35.9 |
| Serine protein kinase* | R298L | 35.1 |
| Ubiquitin conjugating enzyme* | I215L | 24.7 |
| Nudix hydrolase* | D250R | 29.9 |
| Host cell interactions |
|
|
| IAP apoptosis inhibitor* | A224L | 26.6 |
| Bcl 2 apoptosis inhibitor* | A179L | 21.1 |
| Inhibitor of host gene transcription* | A238L | 28.2 |
| C-type lectin-like* | EP153R | 18.0 |
| Similar to HSV ICP34.5 neurovirulence factor | DP71L | 8.5 |
| Nif S-like | QP383R | 42.5 |
| ERV 1-like. Involved in redox metabolism* | B119L | 14.4 |
| Phosphoprotein binds to ribonucleoprotein-K | CP204L | 30.0 |
| Structural proteins and proteins involved in morphogenesis |
|
|
| P22 | KP177R | 20.2 |
| Histone-like | A104R | 11.5 |
| P11.5 | A137R | 21.1 |
| P10 | A78R | 8.4 |
| pA151R. Contains CXXC motif similar to that in thioredoxins. Binds to E248R protein. Not incorporated into virions. Possible component of redox pathway. | A151R | 17.5 |
| P72 major capsid protein. Involved in virus entry | B646L | 73.2 |
| P49. Required for formation of vertices in icosahedral capsid | B438L | 49.3 |
| Chaperone. Involved in folding of capsid. Not incorporated into virions | B602L | 45.3 |
| SUMO-1-like protease. Involved in polyprotein cleavage | S273R | 31.6 |
| pp220 polyprotein precursor of p150, p37, p14 and p34. Required for packaging of nucleoprotein core | CP2475L | 281.5 |
| P32 (P30) phosphoprotein. Involved in virus entry | CP204L | 23.6 |
| pp62 (pp60) polyprotein precursor of p35 and p15 | CP530R | 60.5 |
| P12 attachment protein | O61R | 6.7 |
| P17. Required for progression of precursor membranes to icosahedral intermediates | D117L | 13.1 |
| J5R. Transmembrane domain | H108R | 12.5 |
| P54 (j13L). Binds to LC8 chain of dynein, involved in virus entry. Required for recruitment of envelope precursors to the factory | E183L | 19.9 |
| J18L. Transmembrane domain | E199L | 22.0 |
| P14.5. DNA-binding. Required for movement of virions to plasma membrane | E120R | 13.6 |
| E248R (k2R). Possible component of redox pathway required disulfide bond formation. Structural protein | E248R | 27.5 |
| XP124L. Multigene family 110 member. Contains KDEL ER retrieval sequence and transmembrane domain | MGF 110-4L (XP124L) | 14.2 |
| EP402R. Similar to host CD2 protein. Required for binding red blood cells to infected cells and extracellular virus particles. Glycoprotein inserted into external virus envelope* | EP402R | 45.3 |
| Multigene family members |
|
|
| Multigene family 360 | MGF 360-1L (KP360L) | 41.7 |
|
| MGF 360-2L (KP362L) | 42.6 |
|
| MGF 360-3L (L356L) | 41.7 |
|
| MGF 360-4L (LIS382) | 44.9 |
|
| MGF 360-5L (UP60L) | 7.0 |
|
| MGF 360-6L (LIS375) | 43.9 |
|
| MGF 360-7L (LIS375a) | 44.1 |
|
| MGF 360-8L (J319L) | 31.3 |
|
| MGF 360-9L (A125L) | 14.5 |
|
| MGF 360-10L | 41.6 |
|
| MGF 360-11L | 41.6 |
|
| MGF 360-12L | 41.1 |
|
| MGF 360-13L | 41.0 |
|
| MGF 360-14L | 41.3 |
|
| MGF 360-15R (A276R) | 31.6 |
|
| MGF 360-16R (DP311R) | 35.6 |
|
| MGF 360-17R (DP63R) | 8.4 |
|
| MGF 360-18R (DP148R) | 17.2 |
|
| MGF 360-19R (DP363R) | 42.4 |
|
| MGF 360-20R (DP42R) | 4.9 |
|
| MGF 360-21R | 42.0 |
|
| MGF 360-22R | 41.7 |
| Multigene family 110 | MGF 110-1L (L270L) | 32.4 |
|
| MGF 110-2L (U104L) | 12.2 |
|
| MGF 110-3L (LIS124-1) | 14.3 |
|
| MGF 110-4L (XP124L) | 14.2 |
|
| MGF 110-5L (V82L) | 9.4 |
|
| MGF 110-6L (Y118L) | 13.9 |
|
| MGF 110-7L (LIS137) | 15.9 |
|
| MGF 110-8L (LIS124-2) | 14.9 |
|
| MGF 110-9L (LIS290) | 34.8 |
|
| MGF 110-10L (190-2) | 22.7 |
|
| MGF 110-11L (LIS119-1) | 32.5 |
|
| MGF 110-12L (LIS 119-2) | 12.5 |
|
| MGF 110-13L (LIS 117) | 18.3 |
|
| MGF 110-14L (LIS121-2) | 14.7 |
| Multigene family 300 | MGF 300-1L (J268L) | 31.3 |
|
| MGF 300-2R (J154R) | 17.6 |
|
| MGF 300-3L (J104L) | 12.5 |
|
| MGF 300-4L (J182L) | 21.7 |
| Multigene family 505 | MGF 505-1R | 62.6 |
|
| MGF 505-2R (A489R) | 57.7 |
|
| MGF 505-3R (A280R) | 32.5 |
|
| MGF 505-4R (A505R) | 59.2 |
|
| MGF 505-5R (A498R) | 58.7 |
|
| MGF 505-6R (A518R) | 61.8 |
|
| MGF 505-7R (A528R) | 61.7 |
|
| MGF 505-8R | 61.7 |
|
| MGF 505-9R (A506R) | 59.4 |
|
| MGF 505-10R (A542R) | 59.4 |
|
| MGF 505-11L (DP542L) | 63.1 |
| Multigene family 100 | MGF 100-1R | 15.3 |
|
| MGF 100-2L (DP141L) | 16.8 |
|
| MGF 100-3L (DP146L) | 17.2 |
* The table lists functions of ASFV-encoded proteins. Functions have been demonstrated experimentally for those proteins marked with an asterisk.
Lipids
Enveloped virions contain lipids, including glycolipids and phospholipids such as phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol.
Carbohydrates
One virion protein is glycosylated (pEP402R), and glycolipids are also incorporated into virions. The virus encodes several predicted transmembrane proteins that contain putative N-linked glycosylation sites.
Genome organization and replication
The genomes of different isolates vary in length between 165,795 and 191,036 bp, excluding the terminal inverted repeat sequences, and encode between 151 and 167 protein-encoding ORFs. The ORFs are closely spaced with intergenic distances generally less than 200 bp, and read from both DNA strands. A few intergenic regions contain short tandem repeat arrays.
The primary cell types infected by the virus include those of the mononuclear-phagocytic system, including fixed tissue macrophages and specific lineages of reticular cells. Virus replicates in vitro in macrophages and endothelial cells, and several isolates have been adapted to replicate in tissue culture cell lines. Virus enters cells primarily by clathrin- and dynamin-dependent receptor-mediated endocytosis, and is transported to perinuclear areas associated with the microtubular motor light chain dynein. Early mRNA synthesis begins in the cytoplasm immediately following entry using enzymes and factors packaged in the virus core. Virus DNA replication and assembly take place in perinuclear factory areas. At early times post-infection, virus DNA is detected in the nucleus, suggesting a possible role for nuclear enzymes in initial stages of DNA replication. Head-to-head virus DNA concatemers, which are thought to be replicative intermediates, are detected in the cytoplasm from 6 h post-infection. The mechanism of DNA replication in the cytoplasm is similar to that of viruses in the family Poxviridae.
Virus transcripts are 3′-polyadenylated and 5′-capped. Genes are expressed in an ordered cascade. Early genes are expressed prior to DNA replication; expression of late genes is dependent on the onset of DNA replication. Synthesis of some early genes continues throughout infection. Intermediate genes are expressed late but their expression does not depend on the onset of DNA replication. Promoter elements are relatively short and located immediately upstream from ORFs; transcription start sites are generally a short distance from start codons. Both early and late gene transcripts are of defined length; sequences of seven or more consecutive thymidylate residues in the coding strand are signals for mRNA 3′-end formation.
Several structural proteins are expressed as polyproteins and cleaved at the sequence GlyGlyX. The polyprotein with Mr 220 ×103 is myristylated. Other virus-encoded proteins are modified by phosphorylation (p10 and p32) and N-linked glycosylation. Virus morphogenesis takes place in perinuclear virus factories. Virus factories are surrounded by a vimentin cage and increased numbers of mitochondria. A single lipid membrane thought to be derived from the endoplasmic reticulum is incorporated as an internal lipid membrane in virus particles. The p17 protein is essential for the progression of viral membrane precursors toward icosahedral intermediates. The p54 protein (pE183L) is required for intracellular virus transport and for recruiting envelope precursors to assembly sites. This protein binds to the LC8 component of the dynein motor complex, and this interaction is involved in recruitment to the assembly sites. Formation of the icosahedral capsid occurs on the internal membrane. Assembly of the major capsid protein p72 (pB646L) requires a virus-encoded chaperone pB602L. The pB438L protein is required for formation of the vertices of the icosahedral capsid. The virus genome and enzymes required to initiate infection are packaged into a nucleoprotein core. Processing of the virus polyproteins pp62 and pp220 is essential for core development. Extracellular virus has a loose-fitting external lipid envelope derived by budding through the plasma membrane. Virus is transported to and from sites of assembly on microtubules. The pE120R virion protein is required for virus transport from assembly sites to the plasma membrane.
Antigenic properties
Antibodies induced in pigs that recover from infection with less virulent isolates can neutralize virus infection in pig macrophages and cell lines. This neutralization is effective using virus with a low number of passages in tissue culture but is not as effective against virus with a high number of passages. Serotyping of virus isolates by neutralization has not been carried out since most virulent isolates kill pigs before an effective antibody response is mounted. Virus targets for neutralization in cell culture have been identified using antibodies against proteins p72, p12, p30 and p54, those against p30 inhibiting virus internalization rather than attachment. Virus isolates have been separated into genotypes by sequencing of several genes, but there is no evidence to relate genotype to cross-reactive groups or serotypes.
Biological properties
ASFV infects domestic and wild swine (Sus scrofa domesticus and S. s. ferus), warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus porcus). Disease signs are apparent only in domestic and wild swine. Soft ticks of the genus Ornithodoros are also infected, O. moubata acts as a vector in parts of Africa south of the Sahara and O. erraticus acted as a vector in SW Spain and Portugal. Virus can be transmitted in ticks trans-stadially, and sexual and transovarial transmission has also been demonstrated in O. moubata. Warthogs, bushpigs and swine can be infected by bites from infected ticks. Neither vertical nor horizontal transfer of virus between warthogs is thought to occur. However, transmission between domestic swine can occur by direct contact, by ingestion of infected meat, by fomites, or mechanically by biting flies. Warthogs, bushpigs, wild swine and ticks act as reservoirs of virus. Disease is endemic in domestic swine in many African countries and in Europe in Sardinia. In 2007, African swine fever was introduced to Georgia in the Trans-Caucasus and from there spread to neighbouring countries, including the Russian Federation. ASFV was first reported in Madagascar in 1998 and remains endemic there. Disease was first introduced into Europe in Portugal in 1957 and was endemic in parts of the Iberian peninsula from 1960 until 1995. Sporadic outbreaks have occurred in, and been eradicated from, Belgium, Brazil, Cuba, the Dominican Republic, France, Haiti, Holland and Malta.
ASFV causes hemorrhagic fever in domestic pigs and wild boar. Virus isolates differ in virulence and may produce a variety of disease signs ranging from acute to chronic to inapparent. Virulent isolates may cause 100% mortality in 5–10 days. Moderately virulent isolates have reduced mortality and up to 50% of pigs may recover from infection. Attenuated isolates cause few disease signs. Recovered pigs can remain persistently infected and are protected against challenge with related virulent isolates. CD8+ T cells are required for this protection. Viruses replicate in cells of the mononuclear phagocytic system and reticuloendothelial cells in lymphoid tissues and organs of domestic swine. Cell surface markers expressed from intermediate stages of monocyte–macrophage differentiation are indicators of cell susceptibility to infection. Widespread cell death caused by apoptosis occurs in both T and B lymphocytes in lymphoid tissues and endothelial cells in arterioles and capillaries. This accounts for the lesions seen in acute disease. Disseminated intravascular coagulation develops during the late phase of acute infections, and this may lead to the characteristic hemorrhagic syndrome.
Species demarcation criteria in the genus Asfarviridae
Not applicable.
List of species in the genus Asfivirus
| African swine fever virus | ||
| African swine fever virus Benin97/1 | [AM712239] | (ASFV-Benin97) |
| ASFV-BA71V | [U18466=NC_001659] |
|
| ASFV-Ken | [AY261360] |
|
| ASFV-Mal | [AY261361] |
|
| ASFV-Mku | [AY261362] |
|
| ASFV-OurT88/3 | [AM712240] |
|
| ASFV-Pret | [AY261363] |
|
| ASFV-Teng | [AY261364] |
|
| ASFV-War | [AY261366] |
|
| ASFV-Warm | [AY261365] |
|
| ASFV-E75 | [FN557520] |
|
Species names are in italic script; names of isolates and strains are in roman script. Full genome sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
List of other related viruses which may be members of the genus Asfivirus but have not been approved as species
None reported.
Phylogenetic relationships within the family
Not applicable.
Similarity with other taxa
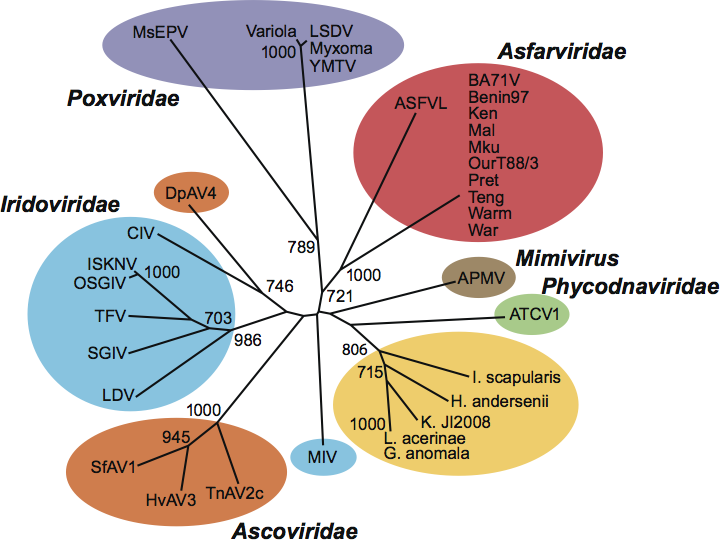
Earlier, ASFV was listed as a member of the family Iridoviridae. As more information was obtained, it was removed from this family. Analysis of the replication strategies and genes encoded have shown that ASFV is related to other viruses in the nucleo-cytoplasmic large DNA virus superfamily, which also includes the families Poxviridae, Iridoviridae, Phycodnaviridae and Mimiviridae (Figure 2). Metagenomic sequencing projects have identified sequences related to ASFV in virus fractions from oceans, sewage and human serum.
Derivation of name
Asfar: from African swine fever and related viruses.
Further reading
Afonso, C.L., Piccone, M.E., Zaffuto, K.M., Neilan, J., Kutish, G.F., Lu, Z., Balinsky, C.A., Gibb, T.R., Bean, T.J., Zsak, L. and Rock, D.L. (2004). African swine fever virus multigene family 360 and 530 genes affect host interferon response. J. Virol., 78, 1858-1864.
Afonso, C.L., Piccone, M.E., Zaffuto, K.M., Neilan, J., Kutish, G.F., Lu, Z., Balinsky, C.A., Gibb, T.R., Bean, T.J., Zsak, L. and Rock, D.L. (2004). African swine fever virus multigene family 360 and 530 genes affect host interferon response. J. Virol., 78, 1858-1864.
Arias, M. and Sanchez-Vizcaino, J.M. (2002). African swine fever. In: A. Morilla, K.-J. Moon and J. Zimmerman (Eds.), Trends in Emerging Viral Infections of Swine. Ames, IO, Iowa State University Press, pp. 119-124.
Chapman, D.A.G., Tcherepanov, V., Upton, C. and Dixon, L.K. (2008). Comparison of the genome sequences of nonpathogenic and pathogenic african swine fever virus isolates. J. Gen. Virol., 89, 397-408.
Galindo, I., Hernaez, B., Diaz-Gil, G., Escribano, J.M. and Alonso, C. (2008). A179l, a viral Bcl-2 homologue, targets the core Bcl-2 apoptotic machinery and its upstream Bh3 activators with selective binding restrictions for Bid and Noxa. Virology, 375, 561-572.
Gomez-Puertas, P., Rodriguez, F., Oviedo, J.M., Brun, A., Alonso, C. and Escribano, J.M. (1998). The african swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology, 243, 461-471.
Granja, A.G., Perkins, N.D. and Revilla, Y. (2008). A238l inhibits NF-ATc2, NF-kappa B, and C-jun activation through a novel mechanism involving protein kinase C-theta-mediated up-regulation of the amino-terminal transactivation domain of P300. J. Immunol., 180, 2429-2442.
Hawes, P.C., Netherton, C.L., Wileman, T.E. and Monaghan, P. (2008). The envelope of intracellular african swine fever virus is composed of a single lipid bilayer. J. Virol., 82, 7905-7912.
Salas, J. and Salas, M.L. (2003). Current perspectives in african swine fever virus infection and evasion of host defenses. Curr. Top. Virol., 3, 155-163.
Suarez, C., Salas, M.L. and Rodriguez, J.M. (2010). African swine fever virus polyprotein pp62 is essential for viral core development. J. Virol., 84, 176-187.
Tulman, E.R., Delhon, G.A., Ku, B.K. and Rock, D.L. (2009). African swine fever virus. In: Van Etten, J.L. (Ed.), Lesser Known Large dsDNA Viruses. Current Topics in Microbiology and Immunology 328, pp. 43-87.
Contributed by
Dixon, L.K., Alonso, C., Escribano, J.M., Martins, C., Revilla, Y., Salas, M.L. and Takamatsu, H.
Figures
Figures
Figure 1 (a) Diagram of extracellular ASFV virions showing nucleoid, matrix or inner core shell, capsid and lipid envelopes. (b) EM image of extracellular virions. Black arrow is outer envelope, white arrow is virus membrane. Bar=200 nm. The preparation method was standard chemical fixation for EM. (c) EM image of intracellular virions. IM=immature virion, M=mature virion. Black arrow is capsid protein, white arrow is virus membrane. Bar=200 nm. Preparation method was high pressure freezing followed by freeze substitution. (d) EM image of intracellular virions. Black arrow is capsid protein, white arrow is virus membrane. Bar=200 nm. Preparation method was thawed cryo-sections stained with uranyl acetate.
(Images kindly provided by Pippa Hawes, Institute for Animal Health, UK.)
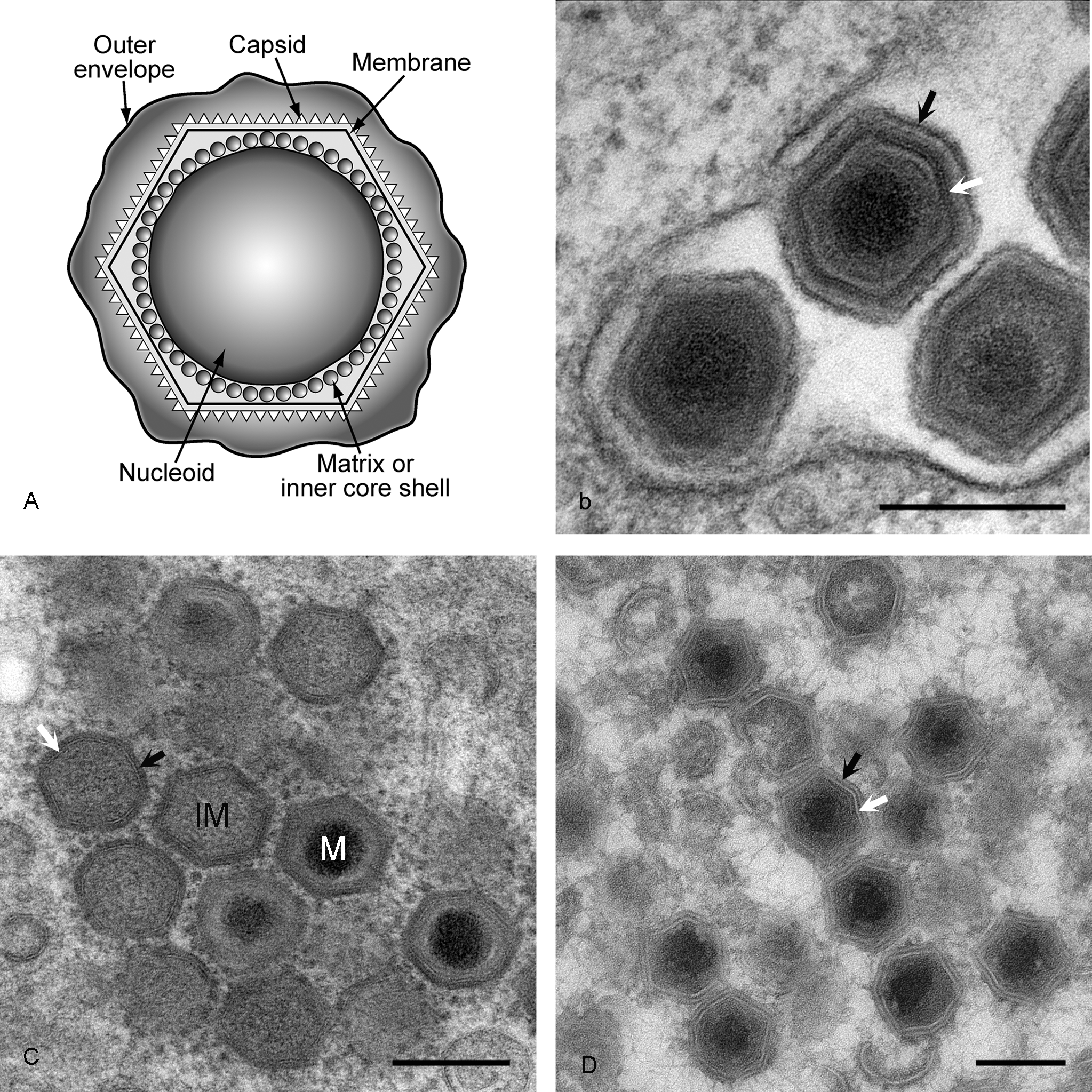
Figure 2 Phylogenetic analysis of ASFV sequences for RNA polymerase, compared to corresponding sequences from dsDNA viruses and non-viral sequences. Sequences are shown in color as follows: asfarviruses, red; mimivirus, brown; poxviruses, purple; phycodnaviruses, green; ascoviruses, orange; iridoviruses, blue; non-viral Blast matches, yellow. Bootstrap values over 65% (>650) are shown. Abbreviations: APMV, Acanthamoeba polyphaga mimivirus; ATCV1, Acanthocystis turfacea chlorella virus 1; CIV, Chilo iridescent virus; DpAV4, Diadromus pulchellus ascovirus 4; G. anomala, Glugea anomala; H. andersenii, Hemiselmis andersinii; HvAV3, Heliothis virescens ascovirus 3; I. scapularis, Ixodes scapularis; ISKNV, infectious spleen and kidney necrosis virus; K. JI2008, Kabatana sp. strain JI2008; L. acerinae, Loma acerinae; LDV, lymphocystis disease virus; LSDV, lumpy skin disease virus; MsEPV, Melanoplus sanguinipesentomopoxvirus; OSGIV, orange-spotted grouper iridovirus; SfAV1, Spodoptera frugiperda ascovirus 1; SGIV, Singapore grouper iridovirus; TFV, tiger frog virus; TnAV2c, Trichoplusia ni ascovirus 2c; YMTV, Yaba monkey tumor virus, Variola, ASFVL African swine fever virus-like sequence.
(Figure redrawn from Loh, J., Zhao, G., Presti, R.M., Holtz, L.R., Finkbeiner, S.R., Droit, L., Villasana, Z., Todd, C., Pipas, J.M., Calgua, B., Girone, R., Wang, D. and Virgin, H.W. (2009). Detection of novel sequences related to African swine fever virus in human serum and sewage. J. Virol., 83, 1301913025.)