Family: Retroviridae
Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release
Virion properties
Morphology
Virions are spherical, enveloped and 80–100 nm in diameter. Glycoprotein surface projections are about 8 nm in length. The internal core constitutes the viral nucleocapsid. The apparently spherical nucleocapsid (nucleoid) is eccentric for members of the genus Betaretrovirus, concentric for members of the genera Alpharetrovirus, Gammaretrovirus, Deltaretrovirus and Spumavirus, and rod or truncated cone-shape for members of the genus Lentivirus.
Structure of retrovirus particles. (Top) Schematic cartoon (not to scale) showing the inferred locations of the various structures and proteins. (Bottom) Panel (A): alpharetrovirus, avian leukosis virus (ALV); type “C” morphology. Panel (B): betaretrovirus, mouse mammary tumor virus (MMTV); type “B” morphology. Panel (C): gammaretrovirus, murine leukemia virus (MLV). Panel (D): deltaretrovirus, bovine leukemia virus (BLV). Panel (E): lentivirus, human immunodeficiency virus 1 (HIV-1). Panel (F): spumavirus, simian foamy virus (SFVcpz(hu); formerly called HFV).
(Courtesy of M. Gonda, reproduced with permission from J.M. Coffin, S.H. Hughes and H. Varmus (Eds.) (1997). Retroviruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.)
Two distinct morphogenic pathways exist. Historically, a nomenclature based on electron microscopy classified members of the Alpharetrovirus and Gammaretrovirus genera, which assemble their immature capsids at the plasma membrane, as C-type viruses. Members of the Betaretrovirus genus in contrast were said to assemble A-type particles (immature capsids) in the cytoplasm which then budded with either a B-type (mouse mammary tumor virus, MMTV) or D-type (Mason-Pfizer monkey virus, MPMV) morphology.
Physicochemical and physical properties
Virion buoyant density is 1.16–1.18 g cm−3 in sucrose. Virion S20,w is approximately 600S in sucrose. Virions are sensitive to heat, detergents and formaldehyde. The surface glycoproteins may be partially removed by proteolytic enzymes. Virions are relatively resistant to UV light.
Nucleic acid
The virus genome characteristic of members of the subfamily Orthoretrovirinae consists of a dimer of linear, positive sense, ssRNA, each monomer 7–13 kb in size. The RNA constitutes about 2% of the virion dry weight. The monomers are held together by hydrogen bonds. Each monomer of RNA is polyadenylated at the 3′ end and has a cap structure (type 1) at the 5′ end. The purified virion RNA is not infectious. Each monomer is associated with a specific molecule of tRNA that is base-paired to a region (termed the primer binding site) near the 5′ end of the RNA and involves about 18 nt at the 3′ end of the tRNA. Other host-derived RNAs (and small DNA fragments) found in virions are believed to be incidental inclusions. The virus genome characteristic of members of the subfamily Spumaretrovirinae is dsDNA, as reverse transcription is a late step in the viral life cycle of these viruses. The exact structure of the DNA has not been determined.
Proteins
Proteins constitute about 60% of the virion dry weight. There are two envelope proteins: SU (surface) and TM (transmembrane) encoded by the viral env gene. Some members of the subfamily Spumaretrovirinae have a third Env protein, LP (leader peptide). There are 3–6 internal, non-glycosylated structural proteins (encoded by the gag gene). These are, in order from the amino terminus, (1) MA (matrix), (2) in some viruses a protein of undetermined function, (3) CA (capsid protein) and (4) NC (nucleocapsid). The MA protein is often acylated with a myristyl moiety covalently linked to the amino-terminal glycine. Other proteins are a protease (PR, encoded by the pro gene), a reverse transcriptase (RT, encoded by the pol gene) and an integrase (IN, encoded by the pol gene). In some viruses, a dUTPase (DU, role uncertain) is also present. Members of the Spumaretrovirinae encode only a single Gag protein which is cleaved once near the carboxyl-terminus in about half of the proteins. The complex retroviruses in the Deltaretrovirus, Epsilonretrovirus, Lentivirus and Spumavirus genera also encode non-structural proteins. Many of these viruses encode transcriptional transactivators, which are required for expression of the LTR promoters, or proteins required for RNA export from the nucleus.
Lipids
Lipids constitute about 35% of the virion dry weight. They are derived from the plasma membrane of the host cell.
Carbohydrates
Virions are composed of about 3% carbohydrate by weight. This value varies, depending on the virus. At least one (SU), and usually both, envelope proteins are glycosylated. Cellular glycolipids and some glycoproteins are also found in the viral envelope
Genome organization and replication
Virions of members of the subfamily Orthoretrovirinae carry two copies of the RNA genome. Infectious viruses have four main genes coding for the virion proteins in the order: 5′-gag-pro-pol-env-3′. Some retroviruses contain genes encoding non-structural proteins important for the regulation of gene expression and virus replication. Others carry cell-derived sequences that are important in pathogenesis. These cellular sequences are inserted either into a complete retrovirus genome (e.g. some strains of Rous sarcoma virus) or in the form of substitutions for deleted viral sequences (e.g. some isolates of murine sarcoma virus). Such deletions render the virus replication-defective and dependent on non-transforming helper viruses for production of infectious progeny. In many cases the cell-derived sequences form a fused gene with a viral structural gene that is then translated into one chimeric protein (e.g. Gag-Onc protein).
Entry into the host cell is mediated by interaction between the virion SU glycoprotein and specific receptors at the host cell surface, resulting in fusion of the viral envelope with the plasma membrane, either directly or following endocytosis. Receptors are cell surface proteins. Many have been identified. For human immunodeficiency virus (HIV), both the CD4 protein, which is an immunoglobulin-like molecule with a single transmembrane region, and a chemokine receptor (CCR5 or CXCR4), which spans the membrane seven times, are required for membrane fusion. The receptors for gammaretroviruses are involved in the transport of small molecules and have a complex structure with multiple transmembrane domains. For the avian leukosis viruses (ALVs), four receptors have been identified. That for subgroup A viruses is a small protein with a single transmembrane domain that is distantly related to a cell receptor for low-density lipoprotein, that for subgroup B viruses is related to the TNF-receptor family of proteins, that for subgroup C viruses is related to the mammalian butyrophilins, and that for subgroup J viruses is the chicken Na+/H+ exchanger protein.
The process of intracellular uncoating of viral particles is not understood. Subsequent early events are carried out in the context of a nucleoprotein complex derived from the capsid.
For members of the subfamily Orthoretrovirinae, replication starts with reverse transcription (by RT) of virion RNA into cDNA using the 3′ end of the tRNA as primer for synthesis of a negative sense cDNA transcript. The initial short product (to the 5′ end of the genome) transfers and primes further cDNA synthesis from the 3′ end of the genome by virtue of duplicated sequences at the ends of the viral RNA. cDNA synthesis involves the concomitant digestion of the viral RNA (RNase H activity of the RT protein). A product of this hydrolysis serves to prime positive sense cDNA synthesis on the negative sense DNA copies. In its final form, the linear dsDNA derived from the viral ssRNA genome contains long terminal repeats (LTRs) composed of unique sequences from the 3′ (U3) and 5′ (U5) ends of the viral RNA flanking a repeated sequence (R) found near both ends of the RNA. The process of reverse transcription is characterized by a high frequency of recombination due to the transfer of the RT from one template RNA to the other. Reverse transcription is thought to follow the same pathway in members of the subfamily Spumaretrovirinae, but the timing is different as it occurs during viral assembly and/or release from the cell. The mechanism of reverse transcription allows for high rates of recombination and genetic diversity for many of the retroviruses. The high rate of genetic variation in vivo can lead to formation of a quasispecies consisting of a large number of genetically diverse virions.
Retroviral DNA becomes integrated into the chromosomal DNA of the host, to form a provirus, by a mechanism involving the viral IN protein. The ends of the virus DNA are joined to cell DNA, involving the removal of two bases from the ends of the linear viral DNA and generating a short duplication of cell sequences at the integration site. Virus DNA can integrate at many sites in the cellular genome. However, once integrated, a sequence is apparently incapable of further transposition within the same cell. The map of the integrated provirus is co-linear with that of non-integrated viral DNA. Integration appears to be a prerequisite for virus replication. Different retroviruses can show distinct preferences in integration site selection, with HIV-1 tending to insert within gene sequences whereas murine leukemia viruses (MLV) prefer regions near the start of transcribed genes.
The integrated provirus is transcribed by cellular RNA polymerase II into virion RNA and mRNA species in response to transcriptional signals in the viral LTRs. In some genera, transcription is also regulated by virus-encoded transactivators. There are several classes of mRNA depending on the virus and its genetic map. An mRNA comprising the whole genome serves for translation of the gag, pro and pol genes (positioned in the 5′-half of the RNA). This results in the formation of polyprotein precursors that are cleaved to yield the structural proteins, PR, RT and IN, respectively. A smaller mRNA consisting of the 5′ end of the genome spliced to sequences from the 3′ end of the genome, and including the env gene and the U3 and R regions, is translated into the precursor of the envelope proteins. In viruses that contain additional genes, other forms of spliced mRNA are also made; all these spliced mRNAs share a common sequence at their 5′ ends. Members of the subfamily Spumaretrovirinae are unique in that they make use of an internal promoter (IP), which is located in the env gene upstream of the accessory reading frames, for transcription of these distal genes. Most primary translation products in retrovirus infections are polyproteins that require proteolytic cleavage before becoming functional. The gag, pro and pol gene products are generally produced from a nested set of primary translation products. For pro and pol, translation involves bypassing translational termination signals by ribosomal frameshifting or by readthrough at the Gag-Pro and/or the Pro-Pol boundaries. Members of the subfamily Spumaretrovirinae synthesize Pol protein from its own mRNA rather than as a Gag-Pol fusion protein.
The retroviral genomic RNA contains sequences of varying lengths, usually located near the 5′ end between U3 and gag, which comprise a packaging signal (Ψ). Ψ is required for efficient encapsidation of the genome into particles, and is generally not present on the subgenomic mRNAs, a notable exception being the alpharetroviruses. In the case of the spumaviruses, Ψ does not appear to be in the 5′ end of the genome. In all cases, Ψ activity is not defined by the primary sequence, but by a complex folded structure.
Capsids assemble either at the plasma membrane (for a majority of the genera), or as intracytoplasmic particles (for members of the genera Betaretrovirus and Spumavirus) and are released from the cell by a process of budding. Budding appears to occur preferentially at specialized membrane microdomains known as lipid rafts. Virions of the spumaviruses and deltaretroviruses are highly cell-associated. Polyprotein processing of the internal proteins occurs concomitant with or just subsequent to the maturation of virions of members of the subfamily Orthoretrovirinae.
Antigenic properties
Virion proteins contain type-specific and group-specific determinants. Some type-specific determinants of the envelope glycoproteins are involved in antibody-mediated virus neutralization. Group-specific determinants are shared by members of a serogroup and may be shared between members of different serogroups within a particular genus. There is evidence for weak cross-reactivities between members of different genera. Epitopes that elicit T-cell responses are found on many of the structural proteins. Antigenic properties are not used in classification of members of the family Retroviridae.
Biological properties
Retroviruses are widely distributed as exogenous infectious agents of vertebrates. Endogenous proviruses that have resulted at some time from infection of germ line cells are inherited as Mendelian genes. They occur widely among vertebrates and can constitute up to 10% of genomic DNA. The vast majority have suffered inactivating mutations and cannot produce infectious virus. A few can exert significant biological effects following activation, either by replication in a manner indistinguishable from exogenous viruses or following recombination with replication-competent virus.
Retroviruses are associated with a variety of diseases. These include: malignancies, including certain leukemias, lymphomas, sarcomas and other tumors of mesodermal origin; mammary carcinomas and carcinomas of liver, lung and kidney; immunodeficiencies (such as AIDS); autoimmune diseases; lower motor neuron diseases; and several acute diseases involving tissue damage. Some retroviruses appear to be non-pathogenic. Transmission of retroviruses is horizontal via a number of routes, including blood, saliva, sexual contact, etc., and via direct infection of the developing embryo, or via milk or perinatal routes. Endogenous retroviruses are transmitted vertically by inheritance of proviruses.
Subfamily Orthoretrovirinae
Genus Alpharetrovirus
Type species Avian leukosis virus
Distinguishing features
Virus particles assemble at the plasma membrane and exhibit a “C-type” morphology. Approximate protein sizes are: MA 19 kDa; p10 10 kDa; CA 27 kDa; NC 12 kDa; PR 15 kDa; RT 68 kDa; IN 32 kDa; SU 85 kDa; and TM 37 kDa. The genome is about 7.2 kb in size (one monomer); its organization is illustrated in Figure 2. There are no known genes additional to gag, pro, pol and env. The tRNA primer is tRNATrp. The LTR is about 350 nt long, of which the U3 region is 250 nt, the R sequence is 20 nt and the U5 region is 80 nt. The viruses have a widespread distribution and include both exogenous (vertical and horizontal transmission) and endogenous viruses of chickens and some other birds. ALV isolates are classified into subgroups (e.g. -A, -J) by their distinct receptor usage. Distantly related endogenous sequences are found in birds and mammals. Virus infections are associated with malignancies and some other diseases such as wasting, and osteopetrosis. Many oncogene-containing members of the genus have been isolated.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome sequence
- Differences in gene product sequences
- Differences in natural host range
- Different oncogenes that may be incorporated.
For example, isolates of avian leukosis virus can be readily distinguished from those of Rous sarcoma virus because they lack oncogene sequences while encoding gag, pol and env. The replication-defective alpharetroviruses can be distinguished from Rous sarcoma virus by the variable deletion of portions of the gag, pol and env genes and the presence of a unique oncogene in each species. Rous sarcoma virus strains encode the src oncogene, whereas avian myeloblastosis virus, for example, encodes the myb oncogene. Host range, defined by SU interaction with a specific receptor, is generally used in defining strains within a species.
List of species in the genus Alpharetrovirus
| Replication-competent, non-oncogene-containing viruses | ||
| Avian leukosis virus |
|
|
| Avian leukosis virus - RSA | [M37980] | (ALV-A) |
| Avian leukosis virus - HPRS103 | [Z46390] | (ALV-J) |
| Replication-competent, oncogene-containing viruses | ||
| Rous sarcoma virus |
|
|
| Rous sarcoma virus (Prague C) | [J02342] | (RSV-Pr-C) |
| Rous sarcoma virus (Schmidt-Ruppin B) | [AF052428] | (RSV-SR-B) |
| Rous sarcoma virus (Schmidt-Ruppin D) | [D10652] | (RSV-SR-D) |
| Replication-defective viruses | ||
| Avian carcinoma Mill Hill virus 2 |
|
|
| Avian carcinoma Mill Hill virus 2 | [K02082] | (ACMHV-2) |
| Avian myeloblastosis virus |
|
|
| Avian myeloblastosis virus | [J02013] | (AMV) |
| Avian myelocytomatosis virus 29 |
|
|
| Avian myelocytomatosis virus 29 | [J02019] | (AMCV-29) |
| Avian sarcoma virus CT10 |
|
|
| Avian sarcoma virus CT10 | [Y00302] | (ASV-CT10) |
| Fujinami sarcoma virus |
|
|
| Fujinami sarcoma virus | [J02194] | (FuSV) |
| UR2 sarcoma virus |
|
|
| UR2 sarcoma virus | [M10455] | (UR2SV) |
| Y73 sarcoma virus |
|
|
| Y73 sarcoma virus | [J02027] | (Y73SV) |
Species names are in italic script; names of strains and isolates are in roman script. Sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
List of other related viruses which may be members of the genus Alpharetrovirus but have not been approved as species
None reported.
Genus Betaretrovirus
Type species Mouse mammary tumor virus
Distinguishing features
Virions of mouse mammary tumor virus (MMTV) exhibit a “B-type” morphology with prominent surface spikes and an eccentric condensed core. Other members of the genus have a “D-type” morphology with fewer dense surface spikes and a cylindrical core. Capsid assembly occurs within the cytoplasm (to yield structures previously termed “A-type” particles) prior to transport to, and budding from, the plasma membrane. Approximate protein sizes are: MA 10 kDa; p21 21 kDa; p8/p12 8 12 kDa; CA 27 kDa; NC 14 kDa; DU 30 kDa; PR 15 kDa; RT 50 kDa; IN 36 kDa; SU 52 kDa; and TM 36 kDa. The genome is 8–10 kb in size (one monomer); its organization in MMTV is illustrated in Figure 3.
In MMTV there are two additional genes: sag, whose product functions as a superantigen, is located at the 3′ end of the genome, overlapping U3 and rem, which encodes an RNA export protein and is translated from a doubly spliced mRNA overlapping the env gene. The sag gene is absent from other members of the genus, but several other viruses encode activities analogous to Rem. The tRNA primer is tRNALys-3 for MMTV and tRNALys-1,2 for other members of the genus. The LTR of MMTV is about 1300 nt long, primarily due to the sag-encoding U3 region of 1200 nt. The R sequence (15 nt) and the U5 region (95–120 nt) are of similar length in all members of the genus.
Viruses assigned to this genus include exogenous (milk-transmitted) and endogenous viruses of mice, as well as exogenous, horizontally transmitted and endogenous viruses of New and Old World primates and sheep. Murine viruses are associated with mammary carcinoma and T-lymphomas, whereas the exogenous primate viruses are associated with immunodeficiency diseases; Jaagsiekte sheep retrovirus is associated with pulmonary cancer of sheep. No oncogene-containing member is known.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome sequence
- Differences in gene product sequences
- Differences in natural host range.
Several primate retroviruses have been described that appear to be divergent members of a single virus that arose from a recombination event in which the env gene of a primate gammaretrovirus was captured. The most divergent of these are the endogenous squirrel monkey retrovirus (SMRV) and langur virus (LNGV), which are unable to infect cells from the primate species of origin. Several serologically distinct strains exist within the species Mason–Pfizer monkey virus. The most divergent of these are the endogenous SMRV and LNGV, although the more closely related isolates Mason–Pfizer monkey virus, simian retrovirus-1 and simian retrovirus-2 are serologically distinct. MMTV is assigned to a separate species because of the unique sag coding region and a widely divergent and distinct env gene. Jaagsiekte sheep retrovirus is also assigned to a separate species on the degree of nucleotide sequence divergence. A closely related virus (enzootic nasal tumor virus, ENTV) induces nasal adenocarcinoma in goats and sheep. Related endogenous proviruses have been identified in other mammalian species (rodents and primates).
List of species in the genus Betaretrovirus
| Jaagsiekte sheep retrovirus |
|
|
| Jaagsiekte sheep retrovirus | [M80216] | (JSRV) |
| (Ovine pulmonary adenocarcinoma virus) |
|
|
| Enzootic nasal tumor virus | [HM104174] | (ENTV) |
| Langur virus |
|
|
| Langur virus |
| (LNGV) |
| Mason–Pfizer monkey virus |
|
|
| Mason–Pfizer monkey virus | [M12349] | (MPMV) |
| Simian retrovirus 1 | [M11841] | (SRV-1) |
| Simian retrovirus 2 | [M16605] | (SRV-2) |
| Mouse mammary tumor virus |
|
|
| Mouse mammary tumor virus | [M15122] | (MMTV) |
| Squirrel monkey retrovirus |
|
|
| Squirrel monkey retrovirus | [M23385] | (SMRV) |
Species names are in italic script; names of isolates are in roman script; names of synonyms are in roman script and parentheses. Sequence accessions [ ] and assigned abbreviations ( ) are also listed.
List of other related viruses which may be members of the genus Betaretrovirus but have not been approved as species
None reported.
Genus Gammaretrovirus
Type species Murine leukemia virus
Distinguishing features
Virions exhibit a “C-type” morphology with barely visible surface spikes. They have a centrally located, condensed core. Capsid assembly occurs at the inner surface of the membrane at the same time as budding. Approximate protein sizes are: MA 15 kDa; p12 12 kDa; CA 30 kDa; NC 10 kDa; PR 14 kDa; RT 80 kDa; IN 46 kDa; SU 70 kDa; and TM 15 kDa. The genome is about 8.3 kb in size (one monomer); its organization is illustrated in Figure 4. The pro-pol region is translated following ribosomal readthrough at the gag gene termination codon. Some gammaretroviruses additionally translate the gag region from an upstream initiation codon, yielding a larger, glycosylated form of Gag protein, glyco-gag. There are no known additional genes. The tRNA primer is tRNAPro (tRNAGlu is found in a few endogenous mouse viruses). The LTR is about 600 nt long, of which the U3 region is 500 nt, the R sequence is 60 nt and the U5 region is 75 nt.
The viruses are widely distributed; exogenous (vertical and horizontal transmission) and endogenous viruses are found in many mammals. The reticuloendotheliosis viruses comprise a few isolates from birds with no known corresponding endogenous relatives. The viruses are associated with a variety of diseases, including malignancies, immunosuppression, neurological disorders. Many oncogene-containing members of the mammalian and reticuloendotheliosis virus groups have been isolated. Viruses resulting from recombination between exogenous and endogenous viruses are frequently encountered.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome sequence and viral oncogenes
- Differences in antigenic properties
- Differences in natural host range
- Differences in pathogenicity
There are mammalian, reptilian and avian (reticuloendotheliosis) viruses. The mammalian viruses include replication-competent viruses, which lack cell-derived oncogenes, and replication defective viruses, which have acquired a variety of oncogenes from their hosts.
Members of the species Murine leukemia virus can be distinguished from isolates of Gibbon ape leukemia virus, for example, by sequence divergence, distinct receptors for virus entry and only limited antigenic cross-reactivity in ELISA assays. Murine sarcoma viruses, which are invariably replication-defective, can be distinguished from the murine leukemia viruses and from one another by the presence of distinct cell-derived oncogenes (e.g. mos in isolates of Moloney murine sarcoma virus and sis in isolates of Woolly monkey sarcoma virus) and the characteristic loss of portions of gag, pol or env. A putative human gammaretrovirus closely related to an endogenous murine retrovirus has recently been discovered, xenotropic MLV-related retrovirus (XMRV).
List of species in the genus Gammaretrovirus
| Replication-competent viruses | ||
| Feline leukemia virus |
|
|
| Feline leukemia virus | [M18247] | (FeLV) |
| Gibbon ape leukemia virus |
|
|
| Gibbon ape leukemia virus | [M26927] | (GALV) |
| Guinea pig type-C oncovirus |
|
|
| Guinea pig type-C oncovirus |
| (GPCOV) |
| Murine leukemia virus |
|
|
| Abelson murine leukemia virus* | [J02009] | (AbMLV) |
| AKR (endogenous) murine leukemia virus | [J01998] | (AKRMLV) |
| Friend murine leukemia virus | [M93134, Z11128] | (FrMLV) |
| Moloney murine leukemia virus | [J02255] | (MoMLV) |
| Porcine type-C oncovirus |
|
|
| Porcine endogenous retrovirus-C | [AM229312] | (PERV-C) |
| Replication-defective viruses | ||
| Finkel-Biskis-Jinkins murine sarcoma virus |
|
|
| Finkel–Biskis–Jinkins murine sarcoma virus | [K02712] | (FBJMSV) |
| Gardner–Arnstein feline sarcoma virus |
|
|
| Gardner-Arnstein feline sarcoma virus |
| (GAFeSV) |
| Hardy–Zuckerman feline sarcoma virus |
|
|
| Hardy-Zuckerman feline sarcoma virus |
| (HZFeSV) |
| Harvey murine sarcoma virus |
|
|
| Harvey murine sarcoma virus |
| (HaMSV) |
| Kirsten murine sarcoma virus |
|
|
| Kirsten murine sarcoma virus |
| (KiMSV) |
| Moloney murine sarcoma virus |
|
|
| Moloney murine sarcoma virus | [J02266] | (MoMSV) |
| Snyder–Theilen feline sarcoma virus |
|
|
| Snyder–Theilen feline sarcoma virus |
| (STFeSV) |
| Woolly monkey sarcoma virus |
|
|
| Woolly monkey sarcoma virus | [J02394] | (WMSV) |
| (Simian sarcoma virus) |
|
|
| Reptilian virus group | ||
| Viper retrovirus |
|
|
| Viper retrovirus |
| (VRV) |
| Avian (reticuloendotheliosis) virus group | ||
| Chick syncytial virus |
|
|
| Chick syncytial virus |
| (CSV) |
| Reticuloendotheliosis virus |
|
|
| Reticuloendotheliosis virus (strain A) | [DQ237900] | (REV-A) |
| Reticuloendotheliosis virus (strain T) |
| (REV-T) |
| Trager duck spleen necrosis virus |
|
|
| Trager duck spleen necrosis virus |
| (TDSNV) |
Species names are in italic script; names of strains and isolates are in roman script. Sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
* A proposal to re-classify AbMLV among the Replication-defective viruses is under preparation.
List of other related viruses which may be members of the genus Gammaretrovirus but have not been approved as species
| Koala retrovirus | [AF151794] | (KoRV) |
Genus Deltaretrovirus
Type species Bovine leukemia virus
Distinguishing features
Virions are similar to those of gammaretroviruses in terms of morphology and assembly. Approximate protein sizes are: MA 19 kDa; CA 24 kDa; NC 12-15 kDa; PR 14 kDa; RT, IN and SU 60 kDa; and TM 21 kDa. The genome is about 8.3 kb in size (one monomer); its organization is illustrated in Figure 5. There are non-structural genes, designated tax and rex, which are involved in regulation of synthesis and processing of virus RNA, in addition to gag, pro, pol and env. The tRNA primer is tRNAPro. The LTR is about 550–750 nt long, of which the U3 region is 200–300 nt, the R sequence is 135–235 nt and the U5 region is 100–200 nt.
The exogenous viruses (horizontal transmission) in this genus are found in only a few groups of mammals. No related endogenous viruses are known. Virus infections are associated with B or T cell leukemias or lymphomas as well as neurological disease (tropical spastic paraparesis or HTLV-associated myopathy) and exhibit a long latency with an incidence of much less than 100%. No oncogene-containing members of this genus have been identified.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome sequence and viral oncogenes
- Differences in antigenic properties
- Differences in natural host range
- Differences in pathogenicity
Primate T-lymphotropic virus species are distinguished primarily on the basis of sequence differences, and each contains several subtypes. All viruses in the genus have a similar coding strategy, but only human T-lymphotropic virus 1 (HTLV-1) has been associated with human disease. HTLV-1 and simian T-lymphotropic virus 1 (STLV-1) are not clustered according to host species but rather according to geographic origin. All HTLV-1 subtypes described so far have most probably originated from separate interspecies transmissions from simians to humans.
List of species in the genus Deltaretrovirus
| Bovine leukemia virus |
|
|
| Bovine leukemia virus | [K02120] | (BLV) |
| Primate T-lymphotropic virus 1 |
|
|
| Human T-lymphotropic virus 1 | [D13784] | (HTLV-1) |
| Simian T-lymphotropic virus 1 | [Z46900] | (STLV-1) |
| Primate T-lymphotropic virus 2 |
|
|
| Human T-lymphotropic virus 2 | [M10060] | (HTLV-2) |
| Simian T-lymphotropic virus 2 | [U90557] | (STLV-2) |
| Primate T-lymphotropic virus 3 |
|
|
| Human T-lymphotropic virus 3 | [DQ093792] | (HTLV-3) |
| Simian T-lymphotropic virus 3 | [Y07616] | (STLV-3) |
Species names are in italic script; names of strains and isolates are in roman script. Sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
List of other related viruses which may be members of the genus Deltaretrovirus but have not been approved as species
| Human T-lymphotropic virus 4 | [EF488483] |
|
| Simian T-lymphotropic virus 5 | [AY590142] |
|
Genus Epsilonretrovirus
Type species Walleye dermal sarcoma virus
Distinguishing features
All members of the genus are exogenous retroviruses. They are complex retroviruses in that their genomes range from 11.7 to 12.8 kb in size and contain 1–3 ORFs, presumably encoding accessory proteins, in addition to those encoding the structural proteins and enzymes of the virion (Figure 6). Orf a is present in all three walleye retroviruses and encodes the rv-cyclin protein, which functions in the control of transcription. The roles of the two additional ORFs (orf b and orf c) in walleye dermal sarcoma virus (WDSV) or the single ORF in snakehead retrovirus have not been determined. The LTRs of the fish retroviruses range from about 500 to 650 nt in length, of which the U3 region is about 450 nt, the R sequence is about 80 nt and the U5 region is about 75 nt. The primer used by the walleye retroviruses is tRNAHis, whereas the snakehead retrovirus uses tRNAArg. Phylogenetic analysis comparing the polymerase region shows that the walleye and perch retroviruses cluster and have diverged significantly from the snakehead retrovirus. Nevertheless, all piscine retroviruses to date appear to group with the mammalian type-C retroviruses.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome sequence and viral oncogenes
- Differences in gene product sequence
- Differences in natural host range
The genus Epsilonretrovirus is comprised of three species of fish retrovirus, which are distinguished from one another on the basis of phylogenetic diversity. In addition, three viruses, snakehead retrovirus (SnRV), salmon swimbladder sarcoma virus (SSSV) and perch hyperplasia virus (PHV), are listed as probable members. As additional fish retroviruses are identified and characterized, both SnRV and SSSV may provide the basis for additional genera (Figure 9). PHV has been sequenced only within the pol gene, and its status remains tentative until further sequence information becomes available.
List of species in the genus Epsilonretrovirus
| Walleye dermal sarcoma virus |
|
|
| Walleye dermal sarcoma virus | [AF033822] | (WDSV) |
| Walleye epidermal hyperplasia virus 1 |
|
|
| Walleye epidermal hyperplasia virus 1 | [AF014792] | (WEHV-1) |
| Walleye epidermal hyperplasia virus 2 |
|
|
| Walleye epidermal hyperplasia virus 2 | [AF014793] | (WEHV-2) |
Species names are in italic script; names of strains and isolates are in roman script. Sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
List of other related viruses which may be members of the genus Epsilonretrovirus but have not been approved as species
| Perch hyperplasia virus |
| (PHV) |
| Snakehead retrovirus | [U26458] | (SnRV) |
| Salmon swimbladder sarcoma virus | [DQ174103] | (SSSV) |
Genus Lentivirus
Type species Human immunodeficiency virus 1
Distinguishing features
Virions have a distinctive morphology with a bar, or cone-shaped core (nucleoid). Viruses assemble at the cell membrane. Approximate protein sizes are: MA – 17 kDa; CA 24 kDa; NC 7–11 kDa; p6 6 kDa; PR 14 kDa; RT 66 kDa; DU (in all except the primate lentiviruses), 14 kDa; IN 32 kDa; SU 120 kDa; and TM 41 kDa. The genome is about 9.3 kb in size (one monomer); its organization is illustrated in Figure 7. Detailed structural data are available for HIV-1 MA, CA, NC, PR, RT, IN, SU and TM.
In addition to the structural gag, pro, pol and env genes, there are additional genes, depending on the virus (e.g. for HIV-1: vif, vpr, vpu, tat, rev and nef) whose products are involved in regulation of synthesis and processing of virus RNA and combating host restriction factors. Most are located 3′ to gag-pro-pol and, at least in part, 5′ to env; one (nef in human immunodeficiency virus 1, HIV-1) is 3′ to env. For other viruses there may be additional non-structural genes (e.g. vpx in human immunodeficiency virus 2, HIV-2). The tRNA primer is tRNALys-3. The LTR is about 600 nt long, of which the U3 region is 450 nt, the R sequence is 100 nt and the U5 region is 80 nt.
The viruses in the genus include exogenous viruses (horizontal and vertical transmission) of humans and many other mammals. Related endogenous viruses have been found in rabbit and lemur. The primate lentiviruses are distinguished by the use of a chemokine receptor and the CD4 protein as receptors and the absence of DU. Some groups have cross-reactive Gag antigens (e.g. ovine, caprine and feline lentiviruses). Viruses related to isolates of Feline immunodeficiency virus have been isolated from other large felids (e.g. puma lentivirus), and antibodies to Gag antigens in lions and other large felids indicate the existence of additional viruses related to FIV and ovine/caprine lentiviruses.
Some lentiviruses are associated with a variety of diseases, including immunodeficiencies, neurological disorders and arthritis, whereas others appear non-pathogenic. No oncogene-containing member of this genus has been isolated.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome and gene product sequences
- Differences in antigenic properties
- Differences in natural host range
- Differences in pathogenicity
Five groups of lentiviruses can be clustered on the basis of the hosts they infect (primates, sheep and goats, horses, cats and cattle). Within the primate lentivirus group, HIV-1 is distinguished from HIV-2, for example, primarily on the basis of sequence divergence that exceeds 50% and the presence of the vpx gene in HIV-2. This reflects the different primate sources for HIV-1 and HIV-2 (chimpanzees and sooty mangabeys, respectively). There is limited cross-reactivity in ELISA tests based on Gag components, but essentially none in those based on env gene products.
List of species in the genus Lentivirus
| Bovine lentivirus group | ||
| Bovine immunodeficiency virus |
|
|
| Bovine immunodeficiency virus | [M32690] | (BIV) |
| Equine infectious anemia virus |
|
|
| Equine infectious anemia virus | [M16575] | (EIAV) |
| Feline lentivirus group | ||
| Feline immunodeficiency virus |
|
|
| Feline immunodeficiency virus (Oma) | [FIU56928] | (FIV-O) |
| Feline immunodeficiency virus (Petuluma) | [M25381] | (FIV-P) |
| Puma lentivirus |
|
|
| Puma lentivirus | [PLU03982] | (PLV-14) |
| Ovine/caprine lentivirus group | ||
| Caprine arthritis encephalitis virus |
|
|
| Caprine arthritis encephalitis virus | [M33677] | (CAEV) |
| Visna/maedi virus |
|
|
| Visna/maedi virus (strain 1514) | [M60609, M60610] | (VISNA) |
| Primate lentivirus group | ||
| Human immunodeficiency virus 1 |
|
|
| Human immunodeficiency virus 1 (four groups, M, N, O and P, are recognized) |
| (HIV-1) |
| Group M (main) has 9 discrete clades (A, B, C, D, F, G, H, J and K) and 15 circulating recombinant forms (RCF). Examples include: |
|
|
| M group Clade A |
|
|
| U455 | [M62320] | (HIV-1.U455) |
| Clade B |
|
|
| ARV-2/SF-2 | [K02007] | (HIV-1.ARV-2/SF-2) |
| BRU (LAI) | [K02013] | (HIV-1.BRU (LAI)) |
| HXB2 | [K03455] | (HIV-1.HXB2) |
| Clade C |
|
|
| ETH2220 | [U46016] | (HIV-1.ETH2220) |
| Clade D |
|
|
| ELI | [X04414] | (HIV-1.ELI) |
| NDK | [M27323] | (HIV-1.NDK) |
| Clade F |
|
|
| 93BR020 | [AF005494] | (HIV-1.93BR020) |
| Clade H |
|
|
| 90CR056 | [AF0055494] | (HIV-1.90CR056) |
| N Group |
|
|
| CM_YBF106 | [AJ271370] | (HIV-1.YBF106) |
| O Group |
|
|
| pCMO2.3 | [AY618990] | (HIV-1.CMO2.3) |
| P Group |
|
|
| RBF168 | [GQ328744] | (HIV-1.RBF168) |
| Human immunodeficiency virus 2 |
|
|
| Human immunodeficiency virus 2 |
| (HIV-2) |
| At least 8 groups, representing independent transmissions from sooty mangabeys, are recognized. |
|
|
| Examples include: |
|
|
| Group A: |
|
|
| BEN | [M30502] | (HIV-2.BEN) |
| ISY | [J04498] | (HIV-2.ISY) |
| ROD | [M15390] | (HIV-2.ROD) |
| Group B: |
|
|
| D205 | [X61240] | (HIV-2.D205) |
| EHOA | [U27200] | (HIV-2.EHOA) |
| UC1 | [L07625] | (HIV-2.UC1) |
| Simian immunodeficiency virus |
|
|
| Simian immunodeficiency virus |
| (SIV) |
| Isolates from at least 40 primate species are known. |
|
|
| Examples include: |
|
|
| African green monkey |
|
|
| African green monkey TYO | [X07805] | (SIV-agm.TYO) |
| African green monkey 155 | [M29975] | (SIV-agm.155) |
| African green monkey 3 | [M30931] | (SIV-agm.3) |
| African green monkey gr-1 | [M58410] | (SIV-agm.gr) |
| African green monkey Sab-1 | [U04005] | (SIV-agm.sab) |
| African green monkey Tan-1 | [U58991] | (SIV-agm.tan) |
| Chimpanzee | [X52154] | (SIV-cpz) |
| Mandrill | [M27470] | (SIV-mnd) |
| Pig-tailed macaque* | [M32741] | (SIV-mne) |
| Red-capped mangabey | [AF028607] | (SIV-rcm) |
| Rhesus macaque* (Maccaca mulatta) | [M19499] | (SIV-mac) |
| Sooty mangabey SIV-H4 | [X14307] | (SIV-smm) |
| Stump-tailed macaque* | [M83293] | (SIV-stm) |
| Sykes monkey | [L06042] | (SIV-syk) |
Species names are in italic script; names of strains and isolates are in roman script. Sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
* Indicates cross-species transmissions of SIV-smm in captivity.
List of other related viruses which may be members of the genus Lentivirus but have not been approved as species
None reported.
Subfamily Spumaretrovirinae
Genus Spumavirus
Type species Simian foamy virus
Distinguishing features
Virions exhibit a distinctive morphology with prominent surface spikes and a central, uncondensed core. Capsid assembly occurs in the cytoplasm prior to budding into the endoplasmic reticulum or from the plasma membrane. Capsid budding requires the presence of Env protein. No cleavage of Gag protein precursors into MA, CA, NC subunits is detectable in infectious virions. The Gag protein is cleaved once near the carboxyl-terminus. Approximate protein sizes are: Gag precursor 71 kDa; N-terminal Gag cleavage product 68 kDa; Pol precursor 127 kDa; RT 85 kDa; IN 40 kDa; Env precursor 130 kDa; SU 80 kDa; TM 48 kDa; LP 18 kDa; Tas 35 kDa; Bet 60 kDa; and Env-Bet fusion protein 170 kDa. The genome is about 11.6 kb in size; its organization is illustrated in Figure 8. The genomic organization is identical to that of other members of the family Retroviridae, as is the mechanism of reverse transcription, which allows inclusion of these viruses into the family. There are two genes (tas and bet) that are expressed in cells in addition to gag, pol and env. Tas is a DNA-binding protein with transactivating function. The exact function of the other accessory protein (Bet) is unknown, but it may be involved in viral latency or act as an inhibitor of the APOBEC3 family. The tRNA primer is tRNALys-1,2. The LTR of primate foamy viruses is about 1770 nt long, of which the U3 region is about 1400 nt, the R region is about 200 nt and the U5 region is 150 nt. In the bovine, equine and feline viruses, the LTR is 950–1400 nt. Spumaviruses make use of two start sites of transcription, R in the LTR and an internal promoter (IP) located upstream of the accessory reading frames within the env gene. The activity of both promoters is Tas-dependent. The additional major criteria distinguishing spumaviruses from members of the other genera are the expression of the Pol protein from a spliced subgenomic RNA and the presence of a large amount of reverse transcribed DNA in the virion, which is required for infection.
Viruses have a widespread distribution, and exogenous spumaviruses are found in many mammals. Phylogenetic analyses using sequences encoding IN are consistent with the hypothesis that simian foamy viruses may have co-evolved with their primate hosts. Human infections have been documented as a result of rare zoonotic transmissions from non-human primates, but human to human spread has not been observed. A distantly related endogenous virus has been reported. Many isolates cause characteristic "foamy" cytopathology in cell culture. No diseases have been associated with spumavirus infections. No oncogene-containing member of the genus has been found.
Species demarcation criteria in the genus
The list of species demarcation criteria is:
- Differences in genome and gene product sequences
- Differences in natural host range
List of species in the genus Spumavirus
| African green monkey simian foamy virus |
|
|
| African green monkey simian foamy virus | [M74895] | (SFVagm) |
| (Simian foamy virus 3, SFV-3) |
|
|
| Macaque simian foamy virus |
|
|
| Macaque simian foamy virus | [X54482] | (SFVmac) |
| (Simian foamy virus 1, SFV-1) |
|
|
| Simian foamy virus |
|
|
| Simian foamy virus, human isolate | [U21247] | (SFVcpz(hu)) |
| (Chimpanzee foamy virus, CFV) |
|
|
| (Human foamy virus, HFV) |
|
|
| (Prototype foamy virus, PFV) |
|
|
| Simian foamy virus, chimpanzee isolate | [L25422] | (SFVcpz) |
| Bovine foamy virus |
|
|
| Bovine foamy virus | [U94514] | (BFV) |
| Equine foamy virus |
|
|
| Equine foamy virus | [AF201902] | (EFV) |
| Feline foamy virus |
|
|
| Feline foamy virus | [Y08851] | (FFV) |
Species names are in italic script; names of isolates are in roman script; names of synonyms are in roman script and parentheses. Sequence accession numbers [ ] and assigned abbreviations ( ) are also listed.
List of other related viruses which may be members of the genus but have not been approved as species
None reported.
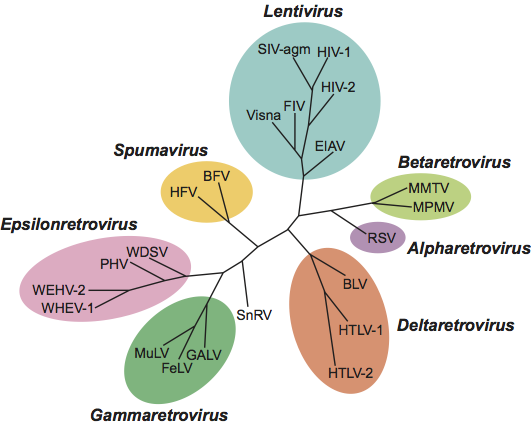
Phylogenetic relationships within the family Retroviridae
See Figure 9.
Similarity with other taxa
There is no nucleotide sequence similarity between members of the families Hepadnaviridae, Caulimoviridae, Pseudoviridae and Metaviridae, as well as with non-viral retroelements. However, there is similarity through the replication strategy with members of the family Hepadnaviridae.
Derivation of names
Lenti: from Latin lentus, “slow”.
Ortho: from Greek orthos, “straight”.
Retro: from Latin retro, “backwards”, refers to the activity of reverse transcriptase and the transfer of genetic information from RNA to DNA.
Spuma: from Latin spuma, “foam”.
Further reading
Coffin, J.M. (1992). Structure and classification of retroviruses. In: Levy, J. (Ed.), The Retroviridae, vol. 1. Plenum Press, New York, pp. 19-50.
Coffin, J.M., Hughes, S.H. and Varmus, H. (eds) (1997). Retroviruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Desrosiers, R.C. (2007). Nonhuman lentiviruses. In: Knipe, D.M. and Howley, P.M. (Eds.), Fields Virology, 5th edn. Lippincott Williams and Wilkins, Philadelphia, PA, pp. 2215-2243.
Doolittle, R.F., Feng, D.F., Johnson, M.S. and McClure, M.A. (1989). Origins and evolutionary relationships of retroviruses. Quart. Rev. Biol., 64, 1-30.
Freed, E.O. and Martin, M.A. (2007). HIVs and their replication. In: Knipe, D.M. and. Howley, P.M (Eds.), Fields Virology, 5th edn. Lippincott Williams and Wilkins, Philadelphia, PA, pp. 2107-2185.
Goff, S.P. (2007). Retroviridae: The retroviruses and their replication. In: Knipe, D.M. and Howley, P.M. (Eds.), Fields Virology, 5th edn. Lippincott Williams and Wilkins, Philadelphia, PA, pp. 1999-2069.
Korber, B.T.M., Foley, B., Leitner, T., McCutchan, F., Hahn, B., Mellors, J.W., Myers, G. and Kuiken, K. (Eds.) (1997). HIV Sequence Compendium – 1997. Los Alamos National Laboratory, Los Alamos, NM.
Lairmore, M.D. and Franchini, G. (2007). Human T-cell leukemia virus types 1 and 2. In: Knipe, D.M. and Howley, P.M. (Eds.), Fields Virology, 5th edn. Lippincott Williams and Wilkins, Philadelphia, PA, pp. 2071-2105.
Leis, J., Baltimore, D., Bishop, J.M., Coffin, J.M., Leissner, E., Goff, S.P., Roszlan, S., Robinson, H., Skalka, A.M., Temin, H.M. and Vogt, V. (1988). Standardized and simplified nomenclature for proteins common to all retroviruses. J. Virol., 62, 1808-1809.
Linial, M.L. (2007). Foamy viruses. In: Knipe, D.M. and Howley, P.M. (Eds.), Fields Virology, 5th edn. Lippincott Williams and Wilkins, Philadelphia, PA, pp. 2245-2261.
Contributed by
Stoye, J.P., Blomberg, J., Coffin, J. M., Fan, H., Hahn, B., Neil, J., Quackenbush, S., Rethwilm, A. and Tristem, M.
Figures
Figure 1 Structure of retrovirus particles. (Top) Schematic cartoon (not to scale) showing the inferred locations of the various structures and proteins. (Bottom) Panel (A): alpharetrovirus, avian leukosis virus (ALV); type C morphology. Panel (B): betaretrovirus, mouse mammary tumor virus (MMTV); type B morphology. Panel (C): gammaretrovirus, murine leukemia virus (MLV). Panel (D): deltaretrovirus, bovine leukemia virus (BLV). Panel (E): lentivirus, human immunodeficiency virus 1 (HIV-1). Panel (F): spumavirus, simian foamy virus (SFVcpz(hu); formerly called HFV).
(Courtesy of M. Gonda, reproduced with permission from J.M. Coffin, S.H. Hughes and H. Varmus (Eds.) (1997). Retroviruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.)
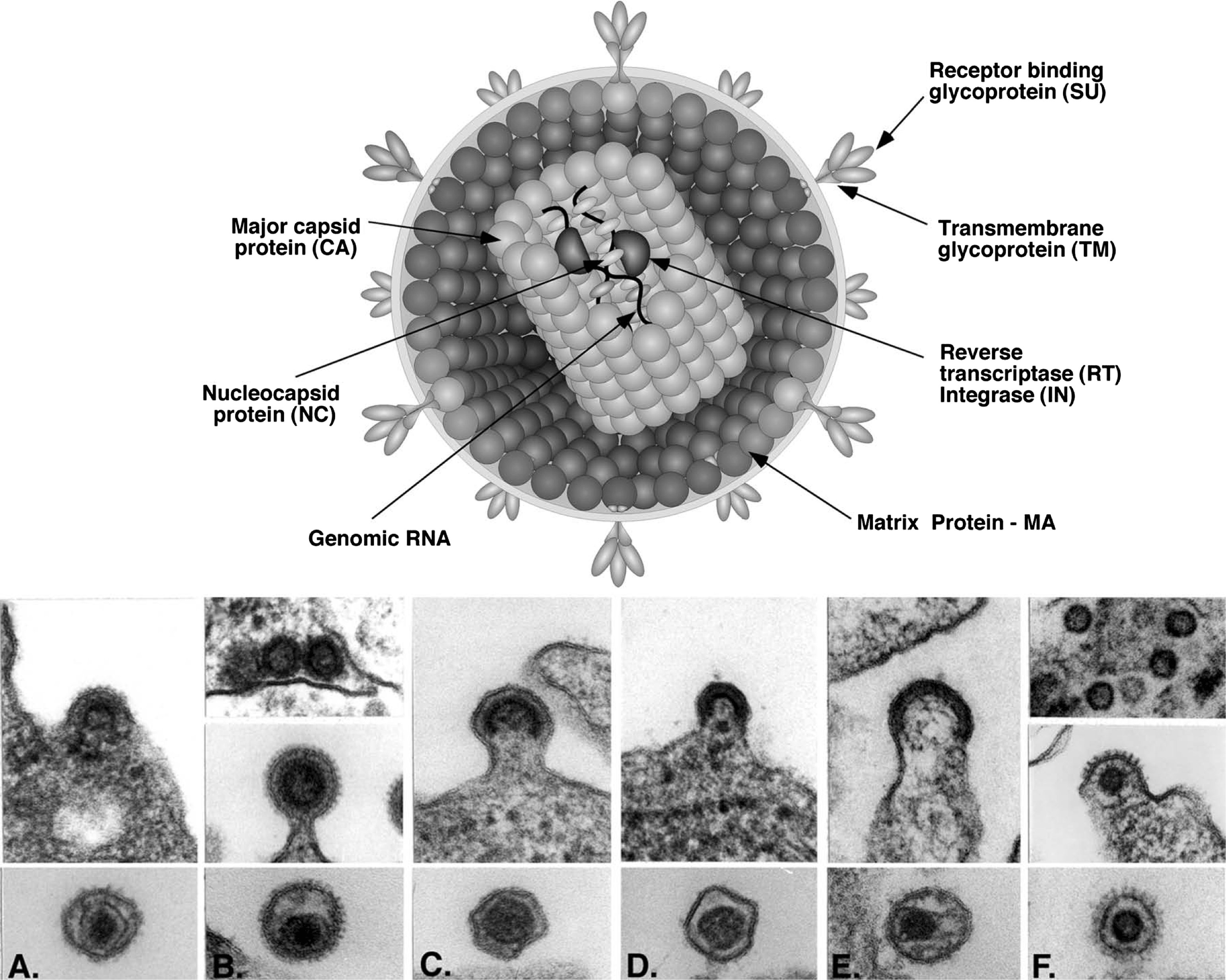
Figure 2 Alpharetrovirus genome expression. The 7.2 kbp avian leukosis virus (ALV) provirus genome is shown, with LTRs, protein-coding regions (gag, pro, pol and env) and transcripts (solid line arrows with protein names added) marked. The arrow between the pro-pol reading frames indicates a ribosomal frameshift.
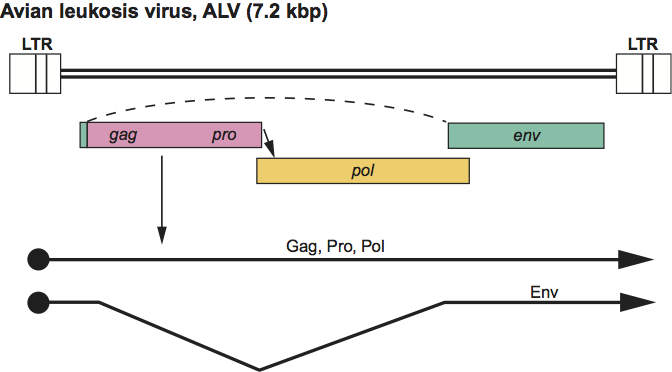
Figure 3 Betaretrovirus genome expression. The 10 kbp mouse mammary tumor virus (MMTV) provirus genome is shown, with LTRs, protein-coding regions (gag, pro, pol, env, sag and rem) and transcripts (solid line arrows with protein names added) marked. The arrows between the gag-pro and pro-pol reading frames indicate ribosomal frameshift sites.
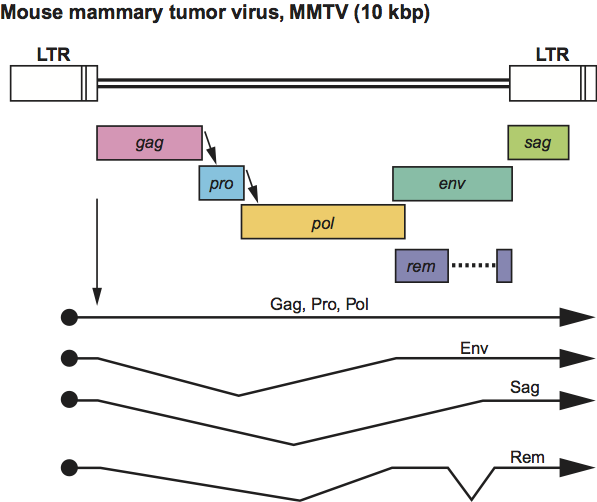
Figure 4 Gammaretrovirus genome expression. The 8.3 kbp murine leukemia virus (MLV) provirus is shown, with LTRs, protein-coding regions (gag, pro, pol and env) and transcripts (solid line arrows with protein names added) marked. The arrow between gag-pro indicates a ribosomal readthrough site.
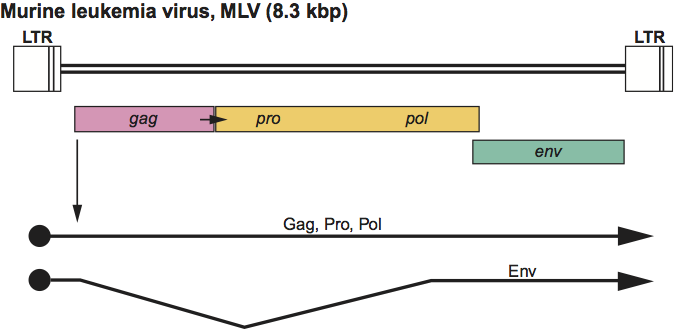
Figure 5 Deltaretrovirus genome expression. The 8.7 kbp human T-lymphotropic virus 1 (HTLV-1) provirus genome is shown, with LTRs, protein-coding regions (gag, pro, pol, env, tax and rex) and transcripts (solid line arrows with protein names added) marked. The arrows between the gag-pro and pro-pol reading frames indicate ribosomal frameshift sites.
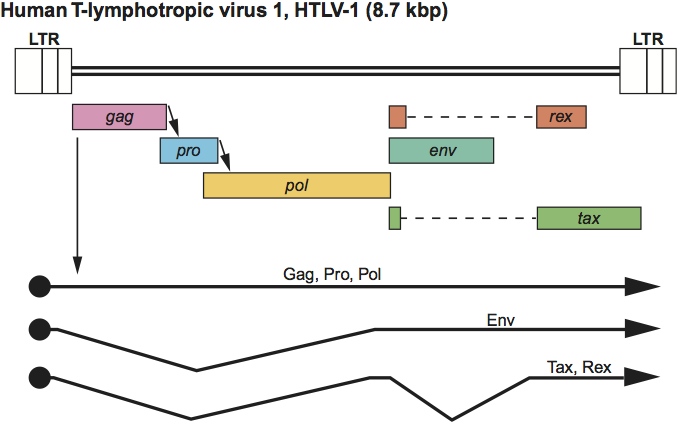
Figure 6 Epsilonretrovirus genome expression. The 12.3 kbp walleye dermal sarcoma virus (WDSV) provirus genome is shown, with LTRs, protein-coding regions (gag, pro, pol, env, orf a, orf b and orf c) and transcripts (solid line arrows with protein names added) marked. The arrow between gag-pro indicates a ribosomal readthrough site.
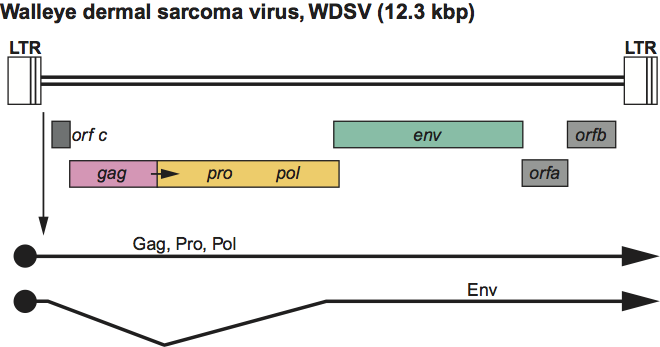
Figure 7 Lentivirus genome expression. The 9.3 kbp human immunodeficiency virus 1 (HIV-1) provirus is shown, with LTRs, protein-coding regions (gag, pro, pol, env, vif, vpr, vpu, tat, rev and nef) and transcripts (solid line arrows with protein names added) marked. The arrow between the gag-pro reading frames indicates a ribosomal frameshift site. The coding regions in other members of the genus may occupy different reading frames.
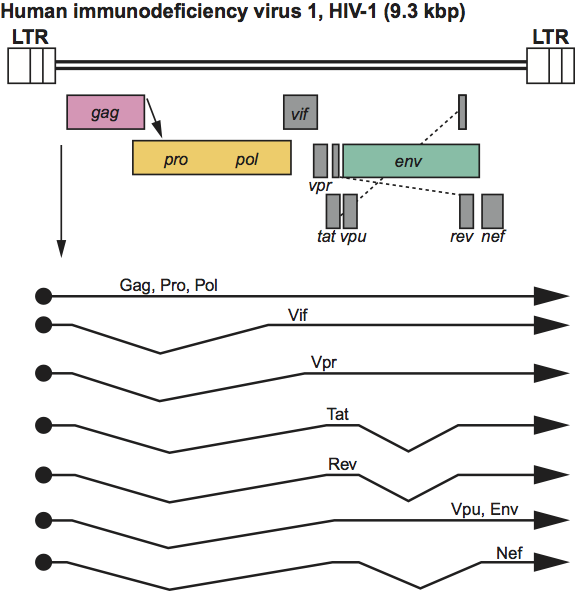
Figure 8 Spumavirus genome expression. The 13.2 kbp simian foamy virus (SFV) provirus is shown, with LTRs, the internal promoter, protein-coding regions (gag, pro, pol, env, tas and bet) and transcripts (solid line arrows with protein names added) marked.
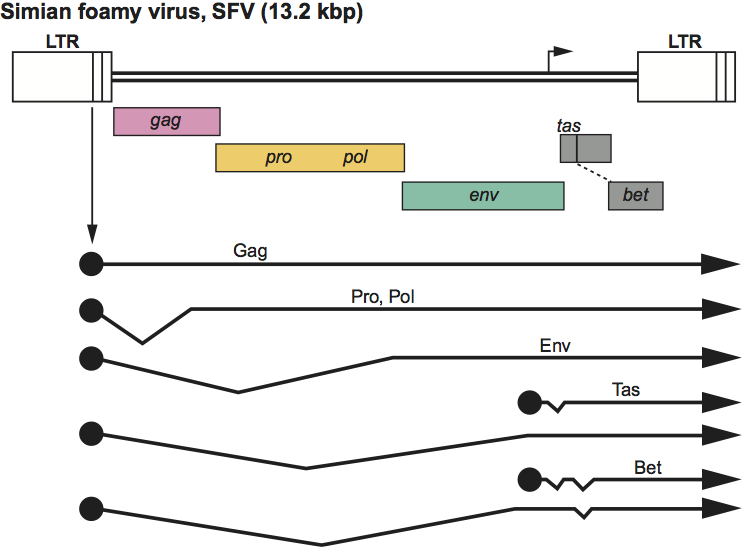
Figure 9 Phylogenetic analysis of conserved regions of the retrovirus polymerase gene (courtesy of Quackenbush, S and Casey, J.). An amino acid sequence alignment was constructed of residues in domains 1 to 4 and part of domain 5 of reverse transcriptase (Xiong, Y. and Eickbush, T.H. (1990). EMBO J., 9, 3353-3362). An unrooted neighbor-joining phylogenetic tree was constructed by using the PHYLIP package (Felsenstein, J. (1995). PHYLIP [Phylogeny Inference Package] Version 3.57c. University of Washington, Seattle.)