Order: Nidovirale/t /t s
Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release
Virion properties
Morphology
The members of the order Nidovirales are enveloped, positive-strand RNA viruses of widely different architecture. Depending on whether the external appearance of the virion or the nucleocapsid is considered, similarities and differences can be discerned (Figure 1).
Figure 1 Schematic structure of particles of members of the order Nidovirales.
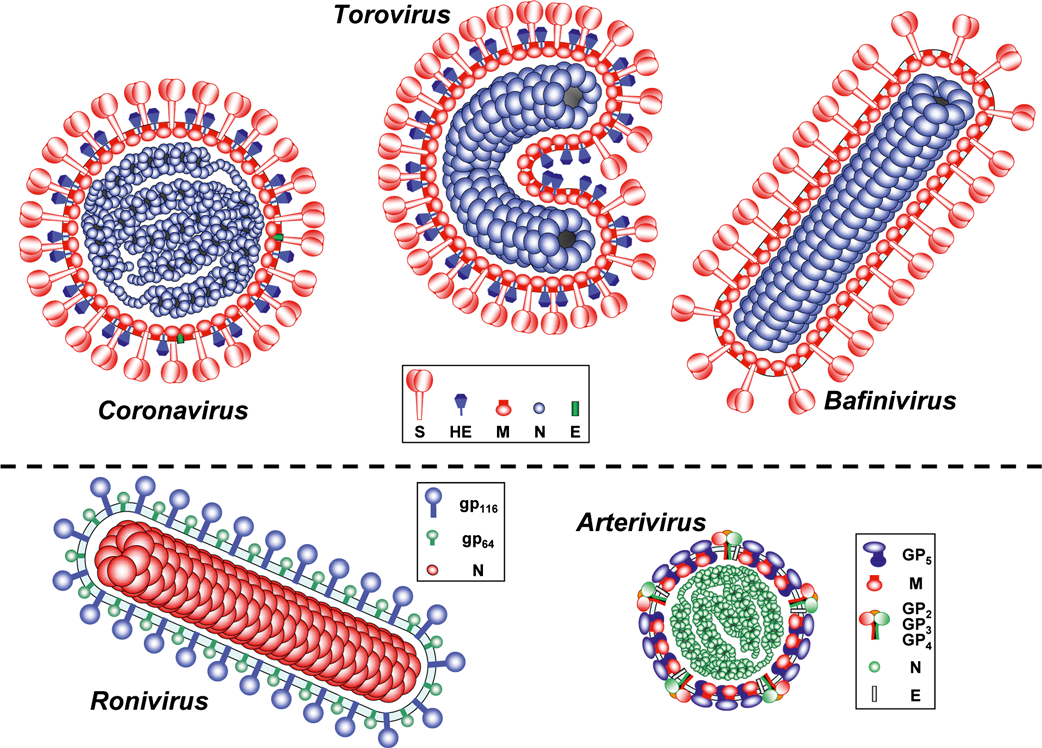
Coronavirinae: As seen in conventional electron micrographs, coronaviruses are roughly spherical enveloped particles, 120–160 nm in diameter, with a characteristic fringe of 15–20 nm petal-shaped surface projections (peplomers). In a subset of betacoronaviruses a second, inner fringe of 5–7 nm surface projections is also seen. Coronavirus (CoV) particles as studied by cryo-electron tomography are homogeneous in size and distinctively spherical (envelope outer diameter 85±5 nm). The envelope exhibits an unusual thickness (7.8±0.7 nm), almost twice that of a typical biological membrane. The nucleocapsid is helical and tightly folded to form a compact structure that tends to closely follow the envelope.
Torovirinae, Torovirus: Toroviruses appear as a mixture of rod-shaped, kidney-shaped and spherical particles. This is, however, most likely an EM artifact. Native torovirus particles are presumably bacilliform with rounded ends, measuring 100–140 nm in length and 35–42 nm in width (envelope outer dimensions). Virions carry two types of surface projections that in size and shape closely resemble those of (beta)coronaviruses. The most distinctive virion element, the core, is a flexible and seemingly hollow tube of helical symmetry (periodicity ca. 4.5 nm), about 100 nm in length and about 23 nm across with a central channel of about 10 nm in diameter.
Torovirinae, Bafinivirus: Bafiniviruses (bacilliform fish nidoviruses) are 130–160×37–45 nm in dimension (excluding spikes) with a rod-like nucleocapsid in the form of a rigid cylinder (120–150×19–22 nm with a central channel of 2–5 nm). The virion envelope is studded with 20–25 nm coronavirus-like peplomers.
Roniviridae: Roniviruses (rod-shaped nidoviruses) are also bacilliform in shape, 150–200 nm in length and about 45 nm in diameter, and contain a tightly coiled nucleocapsid with a diameter of about 25 nm and a 5–7 nm helical periodicity. The ronivirus envelope bears spikes, but smaller in size than those of coronaviruses, projecting approximately 11 nm from the surface.
Arteriviridae: Arterivirus virions are significantly smaller than those of the other nidoviruses, spherical or egg-shaped and with a seemingly isometric core that contains the genome. Complete particles and nucleocapsids, as measured by cryo-EM, average 54 nm and 39 nm in diameter, respectively, with core and envelope separated by a 2–3 nm gap. Three-dimensional reconstructions, based on cryo-EM tomography, suggests that the core might consist of a helical nucleocapsid wrapped into a hollow ball. No spikes are obvious on the arterivirus surface, but a surface pattern of relatively small and indistinct projections has been observed.
Physicochemical and physical properties
The coronavirus virion Mr is 400×106, the buoyant density in sucrose is 1.15–1.20 g cm−3, the density in CsCl is 1.23–1.24 g cm−3, and the virion S20,W is 300–500S. Torovirus and bafinivirus virions have buoyant densities in sucrose of 1.14–1.18 and 1.17–1.19 g cm−3, respectively. Arterivirus virion buoyant density is 1.13–1.17 g cm−3 in sucrose and 1.17–1.20 g cm−3 in CsCl; virion S20,W is 200 to 300S. Ronivirus virion buoyant density in sucrose is 1.18–1.20 g cm−3. Nidovirus virions are sensitive to heat, lipid solvents, non-ionic detergents, formaldehyde, oxidizing agents and UV irradiation.
Nucleic acid
The nidoviruses genome is an infectious, linear, positive sense RNA molecule, which is capped and polyadenylated. Based on the genome size, two groups – large and small nidoviruses – can be distinguished. The genomes of the large nidoviruses are well over 25 kb in length with size differences in the 5 kb range: 26.4–31.7 kb (Coronavirus), 28–28.5 kb (Torovirus), about 26.6 (Bafinivirus), and 26.2– 26.6 kb (Okavirus). The small nidoviruses include a single family (Arteriviridae) with genomes from 12.7– 15.7 kb in length. Members of the families Corona- and Roniviridae are the largest RNA viruses known to date. Complete genome sequences are available for representatives of all seven nidovirus genera.
Proteins
Although the structural proteins of the nidoviruses are generally functionally equivalent, there is no firm indication that any single protein species is evolutionary conserved across all of the families. The virion proteins typical for each of the five main nidovirus taxa are listed in Table 1.
Table 1 Structural proteins of nidoviruses: acronyms and sizes (in amino acid residues). Boxed proteins are believed to be evolutionarily related
| Proteina |
| Coronavirus | Torovirus | Bafinivirus | Okavirus | Arterivirus |
| Spike glycoprotein | S | 1128–1472 | 1562–1584 | 1220 | - | - |
| Large spike glycoprotein | gp116 | - | - | - | 873c–899 | - |
| Small spike glycoprotein | gp64 | - | - | - | 539 | - |
| Minor surface glycoprotein | GP2 | - | - | - | - | 227–249 |
|
| GP3 | - | - | - | - | 163–256 |
|
| GP4 | - | - | - | - | 152–183 |
| Major surface glycoprotein | GP5 | - | - | - | - | 199–278 |
| Membrane protein | M | 218–263 | 233 | 227 | - | 162–174 |
| Nucleocapsid protein | N | 349–470 | 159–167 | 161 | 144–146 | 110–128 |
| Envelope protein | E | 74–109 | - | - | - | 67–80 |
| Hemagglutinin-esterase protein | HE | 386–440b | 416–430 | - | - | - |
a Only proteins typical for each lineage are listed; for some CoVs additional, virus species-specific accessory envelope proteins have been described
b Only found in a cluster of betacoronaviruses (“phylogroup A”, Betacoronavirus 1, Murine coronavirus, Human coronavirus HKU-1).
c Size predicted for gill-associated virus gp116 protein.
Members of the family Coronaviridae generally possess three or four envelope proteins. The most abundant one (at least in corona- and toroviruses) is the membrane (M) protein. Though different in sequence, the M proteins of corona-, toro- and bafiniviruses are alike in size, structure and presumably also in function. They have a similar triple-spanning membrane topology with a short amino terminus located on the outside of the virion, and a long C-terminal endodomain, comprising an amphiphilic region and a hydrophilic tail. The amphiphilic segment is believed to associate with the inner leaflet of the membrane to form a matrix-like lattice, which would explain the remarkable thickness of the coronavirus envelope as observed by cryo-electron tomography. Of note, in transmissible gasteroenteritis virus of swine (Alphacoronavirus 1), a second population of M proteins adopting an Nexo-Cexo topology in the viral envelope has been described.
The spike (S) proteins of corona-, toro- and bafiniviruses are exceptionally large type I membrane glycoproteins (1200–1600 aa residues), heavily N-glycosylated and with features characteristic of class I fusion proteins. Remote but significant sequence similarity among S proteins of toro-, bafini- and (to lesser extent) coronaviruses suggests common ancestry and similarity in structure and function, i.e. receptor-binding and membrane fusion. With few exceptions, the S proteins become proteolytically cleaved during virion biogenesis into subunits S1 and S2 that remain associated. Coronavirus S proteins assemble into homotrimers and this most likely also occurs with the S proteins of toro- and bafiniviruses. The bulbous membrane-distal part of the peplomers, comprising the receptor-binding domains, are largely composed of S1 subunits, whereas the C-terminal S2 subunits form a membrane-anchored stalk. Heptad repeat regions in S2 are assumed to drive membrane fusion during entry by undergoing a series of conformational changes culminating in a six-helical bundle. In the primary structure, the N-terminal repeat, HR1, is located immediately downstream of a predicted internal fusion peptide and the other repeat, HR2, immediately upstream of the transmembrane domain.
Coronaviruses code for a small envelope protein (E), a pentameric integral membrane protein exhibiting ion channel and/or membrane permeabilizing (viroporin) activities. With around 20 copies per particle, the E protein is only a minor structural component. Although its precise function remains to be defined, the E protein has been implicated in virion morphogenesis and identified as a virulence factor for Severe Acute Respiratory Syndrome (SARS)-CoV. So far, no E homologs have been identied in toro- and bafiniviruses.
A subset of betacoronaviruses (Betacoronavirus 1, Murine coronavirus and Human coronavirus HKU1), and all toroviruses known to date, code for an additional homodimeric type I membrane glycoprotein, the hemagglutinin-esterase (HE), that mediates reversible virion attachment to O-acetylated sialic acids by acting both as a lectin and as a sialate-O-acetylesterase. Corona- and torovirus HEs share 30% sequence identity and thus are far more closely related to each other than are the S and M proteins of these nidovirus lineages. While the latter two protein species might have been encoded in the last common ancestor of the Corona- and Torovirinae lineages, the HE proteins must have been acquired relative recently. Originating from a hemagglutinin-esterase fusion protein resembling that of influenza C virus, the HEs appear to have been introduced into the betacorona- and torovirus proteomes independently (i.e. through two separate horizontal gene transfer events) well after the Corona–Torovirinae split, and in the case of the coronaviruses even after their separation into alpha-, beta- and gammacoronaviruses.
The nucleocapsid (N) proteins of corona- and toroviruses are highly basic, RNA-binding phosphoproteins, involved in encapsidation and packaging of the genome. However, as demonstrated for coronaviruses, N proteins might also play essential roles in RNA synthesis and translation, exhibit RNA chaperone activity and act as antagonists of interferon type I. With molecular masses of about 18 kDa, the torovirus N and proposed bafinivirus N proteins are less than half the size of their coronavirus equivalents. The structural, functional and evolutionary relationships between these protein species remain to be established.
The structural proteins of arteriviruses are apparently unrelated to those of the other members of the order Nidovirales. The nucleocapsid contains a single protein species, N. In equine arteritis virus (EAV) and porcine reproductive and respiratory syndrome virus (PRRSV), six envelope proteins have been identified, each essential for virion infectivity. The non-glycosylated membrane protein (M) is thought to span the membrane three times and thus to structurally resemble the M protein of corona- and toroviruses. It forms a disulfide-linked heterodimer with the major glycoprotein (GP5 for EAV, PRRSV and lactate dehydrogenase-elevating virus, LDV; GP7 in simian hemorrhagic fever virus, SHFV), which is also a putative triple-spanning membrane protein. Viral glycoproteins GP2, GP3 and GP4 are minor virion components and form heterotrimers. The remaining envelope protein, E (for envelope), is small, hydrophobic and non-glycosylated, and believed to function as an ion-channel protein. LDV virion composition has been studied in less detail, but is likely similar to that of EAV and PRRSV. Remarkably, SHFV may possess up to three additional envelope proteins.
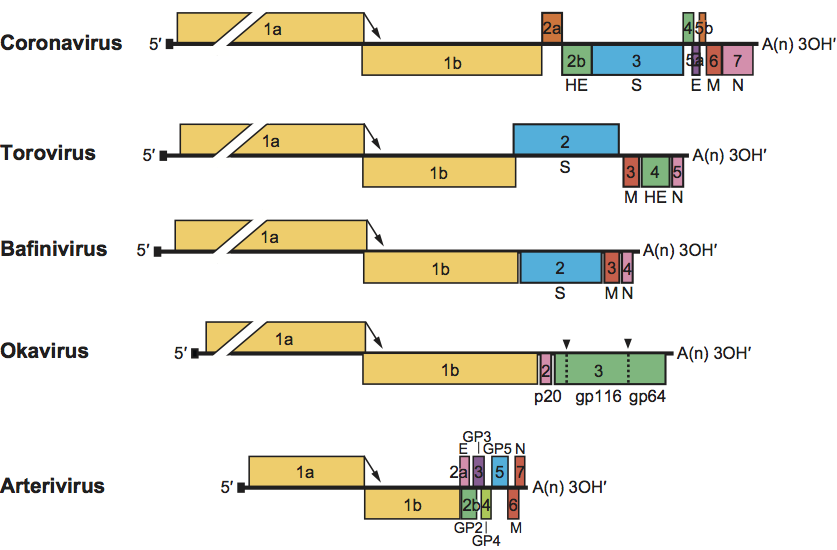
Ronivirus structural proteins have been studied only for yellow head virus (YHV). Virions contain a highly basic nucleoprotein species (p20) and two envelope glycoprotein species (gp116 and gp64) that form the prominent peplomers on the virion surface. Both gp116 and gp64 are encoded by the ORF3 gene and generated from a long (1640–1666 aa residues) precursor glycopolyprotein (pp3) by post-translational processing at two internal signal peptidase type-1 sites (Figure 2). They are not linked by intramolecular disulfide bonds and are anchored in the envelope by either one (gp64) or two (gp116) hydrophobic C-terminal transmembrane domains. Processing of pp3 would also yield an N-terminal product of about 25 kDa, a putative triple-spanning membrane protein, the fate and function of which are not known.
Figure 2 Schematic representation of the genome structure of members of the order Nidovirales (from top to bottom: murine coronavirus, bovine torovirus, white bream virus, gill-associated virus, equine arteritis virus). Note that, in coronaviruses, the 3′ genome organization and the complement of accessory genes can differ even among members of the same genus. ORFs are represented by boxes. Untranslated sequences are indicated by solid lines. The ribosomal frameshift sites in ORF1 (located at the 1a-1b junction and indicated by arrows) are aligned. Numbers refer to the mRNA species from which the ORFs are expressed. The proteins encoded by the ORFs are indicated above or below. Signal peptidase type-1 cleavage sites in okavirus precursor glycopolyprotein pp3 are indicated by dashed lines and arrowheads. The 5′ leader sequences are depicted by a small black box. Poly(A) tails are indicated by A(n). S, spike protein; M, membrane protein; E, envelope protein; N, nucleocapsid protein; HE, hemagglutinin-esterase protein.
Lipids
Nidoviruses have lipid envelopes, which are commonly acquired by budding at membranes of the endoplasmic reticulum, intermediate compartment and/or Golgi complex. Coronavirus S and E proteins are palmitoylated; the arterivirus E protein is myristoylated.
Carbohydrates
Coronavirus S and HE proteins are heavily glycosylated and contain multiple N-linked glycans (20–35 and 5–11, respectively). The M protein of coronaviruses contains a small number of either N- or O-linked glycans, depending on the virus species, located near the amino-terminus. Coronavirus E proteins are not glycosylated. Torovirus S and HE proteins are also heavily N-glycosylated (19–25 and 7–13 glycans, respectively); the M protein is not glycosylated, however. Bafinivirus structural proteins have not been characterized in great detail. The S and M proteins appear to be glycosylated. The S protein binds lectins and likely contains α-mannose. The gp116 and gp64 proteins of ronivirus YHV contain 6 and 3 N-linked glycans, respectively. In arteriviruses, GP2, GP3, GP4 and GP5 contain N-linked glycans. GP5 of EAV, LDV, and PRRSV are modified by heterogeneous N-acetyl lactosamine addition. Due to extensive and heterogeneous glycosylation, GP5 is of highly variable size (between 26 and 42 kDa). The M and E proteins are not glycosylated.
Genome organization and replication
Nidovirus replication takes place in the cytoplasm of infected cells and proceeds through the synthesis of minus-strand intermediates. RNA synthesis is catalyzed by an as yet poorly characterized replication–transcription complex, composed of viral and host proteins and presumably associated (at least in corona- and arteriviruses) with a network of modified intracellular membranes, which is derived from the ER and includes unusual double-membrane vesicles.
Genome organization
Despite considerable differences in genome size and gene composition, nidoviruses are remarkably similar in their genome organization (Figure 2). The 5′-most two-thirds of the genome characteristically comprises two large, partially overlapping ORFs, designated 1a and 1b, that constitute the replicase gene and together encode a collection of enzymes that are part of the replication complex per se or control its composition and functioning (see section on replicase). The virion RNA functions as mRNA (mRNA1) for ORFs 1a and 1b, but the expression of the latter requires a programmed ribosomal frameshift. Translation of ORF1a yields polyprotein pp1a. In 20–30% of the cases, ribosomes do not reach the ORF1a termination codon, but slip at the ORF1a/1b overlap and shift register to the 21 reading frame to continue translation into ORF1b. The ribosomal frameshift occurs within a specific seven-nucleotide “slippery” sequence, upstream of a pseudoknot structure, and gives rise to a 3′-extended fusion polyprotein, pp1ab. The replicase polyproteins are processed by several virus-encoded proteases to more than a dozen mature products, including the key replicative enzymes/proteins of the virus (further detailed below).
Downstream of the replicase gene there are from three (Okavirus) to up to 12 (Coronavirinae) ORFs that encode a set of structural proteins typical for the subfamily and/or genus, and, at least for coronaviruses, a variety of “accessory” proteins that may be virus species or even subspecies-specific. These 3′-proximal ORFs are expressed from a 3′-coterminal nested set of dedicated subgenomic (sg) mRNAs, the number of which ranges from two in okaviruses to up to at least eight in certain coronaviruses. All mRNA species, except the smallest ones, are structurally polycistronic. As a rule, however, translation is restricted to the 5′-most ORF(s) not present in the next smaller mRNA of the set; downstream ORF(s) remain translationally silent.
Nidovirus transcription units (i.e one or more ORFs expressed from a single mRNA species) are generally preceded in the genome by short conserved sequence elements commonly termed “transcription-regulating sequences” (TRSs) in corona-, arteri- and bafiniviruses and putative terminator/promoter elements (TPs) in toroviruses. As toro- and roniviruses differ in their transcription mechanism from the other nidoviruses (see below), TRSs and TPs are not functionally equivalent.
The replicase gene
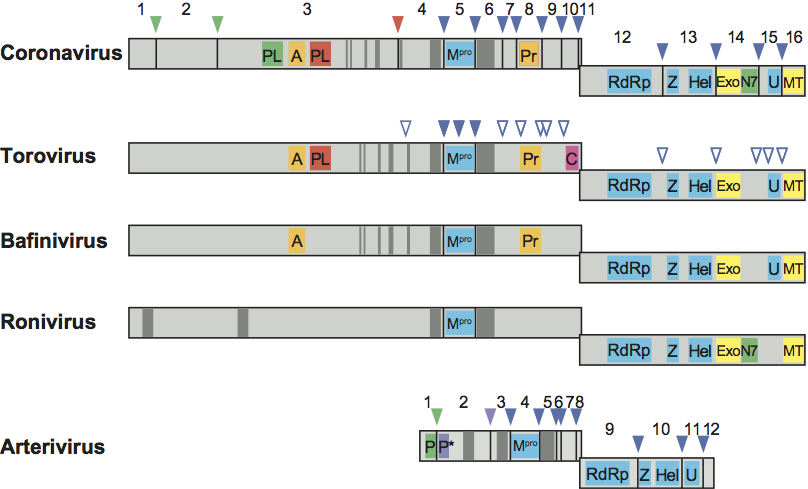
Expression of the replicase ORF1a/1b gene yields two huge polyproteins, pp1a and pp1ab. These giant proteins (ranging in size from the approximately 2000-aa pp1a of arteriviruses to the >7000-aa pp1ab of coronaviruses) have not been observed in infected cells. Their processing by viral proteinases is believed to occur both cotranslationally and posttranslationally, yielding more than a dozen mature proteins (13 in arteriviruses and 15 or 16 in coronaviruses) and an as-yet unknown number of functional intermediates. In arteriviruses and coronaviruses, from one to three (and possibly four) papain-like cysteine proteases (PLpro in coronaviruses, PCP and CP in arteriviruses) control the proteolytic processing of the N-terminal part of pp1a/pp1ab at 2-4 sites. A protease with a chymotrypsin-like fold (known as 3CLpro or “main” protease, Mpro; also designated as serine protease SP in arteriviruses) is responsible for the processing of the remaining largest part of pp1a/pp1ab at 8-11 conserved cleavage sites. Nidovirus pp1a/pp1ab processing products, generally referred to as the nonstructural proteins (nsp’s), are numbered according to their position (from N- to C-terminus) in the viral polyproteins (nsp1 to nsp12 in arteriviruses and nsp1 to 16 in coronaviruses; Figure 3). In some cases, alternative names are used to refer to functional domain(s) present in these nsp’s, especially in cases where the domains are conserved across nidovirus (sub)families and mediate specific functions and/or enzymatic activities.
Despite a more than two-fold difference in size between the replicase genes of arteriviruses and other nidoviruses, a common backbone of conserved domains can be discerned. Sequence alignments and phylogenetic analyses suggest that the conservation of functional domains in the replicase polyproteins is the result of a continuous evolution from a common nidovirus ancestor. Activities and functions have been identified for many of the conserved replicase domains and the corresponding cleavage products. Replicase subunits conserved across all nidoviruses include (from N- to C-terminus): (i) a chymotrypsin-like protease (3C-like or main protease; 3CLpro, Mpro) that is flanked by two hydrophobic transmembrane domains (tm-Mpro-tm) in the viral polyprotein and has a substrate specificity resembling that of picornavirus 3C proteases, (ii) a large RdRp, (iii) a 5′-to-3′ helicase domain containing a putative multinuclear Zn-finger-like domain at its N-terminus (Zn-HEL). Some replicase subunits are present only in a subset of nidoviruses. A nidoviral endoribonuclease specific for uridylate (NendoU) was long considered to be shared by all nidoviruses and to represent a unique diagnostic molecular marker that would distinguish the members of this order from all other RNA viruses known to date. However, a very recent study shows roniviruses to lack this domain. A 3’-to-5’ exoribonuclease (ExoN) and ribose-2’-O-methyltransferase (O-MT) are conserved in the large nidoviruses, but not in arteriviruses. An ADP-ribose-1”-phosphatase (ADRP, also called macrodomain) and a noncanonical “secondary” RdRp with possible primase activity (coronavirus nsp8), have been (tentatively) mapped only in members of the family Coronaviridae. A guanine N7 methyltransferase, recently identified in coronaviruses, appears to be conserved in roniviruses. The conservation of key proteolytic and RNA-processing enzymes in the main nidovirus lineages is summarized in Figure 3.
The N-terminal half of pp1ab is quite variable among nidoviruses, even among members of the same genus. This variability contributes significantly to the major size differences between the genomes of large and small nidoviruses. A comparison between the coronavirus and arterivirus N-terminal pp1a/pp1ab sequences does not yield significant sequence similarities beyond the conservation of active sites of papain-like proteases.
Figure 3 Schematic representation of the domain organization of the replicase polyproteins pp1a and pp1ab of representative viruses from the five main nidovirus taxa (from top to bottom: murine coronavirus, bovine torovirus, white bream virus, gill-associated virus, equine arteritis virus). The position of the ribosomal frameshift site was used to align the polyprotein representations. Cleavage sites in pp1a and pp1ab of papain-like proteinases (PL, P) or of the 3C-like main protease (Mpro) are indicated by color-coded arrowheads; open arrowheads indicate predicted Mpro cleavage sites in the torovirus replicase polyproteins. The processing end-products (nonstructural proteins) of corona- and arterivirus polyproteins are numbered; conserved domains are highlighted as follows: PL, coronavirus papain-like proteinase; P and P*, arterivirus papain-like cysteine proteinases PCP and CP, respectively; A, ADP-ribose-1″-phosphatase (macrodomain); Mpro, 3C-like main protease; Pr, noncanonical RNA-dependent RNA polymerase, putative primase; C, cyclic nucleotide phosphodiesterase domain; RdRp, RNA-dependent RNA polymerase; Z, zinc-binding domain; Hel, helicase domain; Exo, 3′-to 5′ exoribonuclease domain; N7, guanine-N7-methyltransferase; U, nidoviral uridylate-specific endoribonuclease (NendoU); MT, ribose-2′-O-methyltransferase domain.
Synthesis of genomic and subgenomic rnas
Genome replication and sg mRNA synthesis (“transcription”) proceed through minus-strand intermediates. The genome serves as a template for the synthesis of full-length minus-strand RNA, from which in turn new genome copies are produced, but it is also believed to be the template for the synthesis of sg minus-strand RNA species (vide infra). The synthesis of viral RNAs is highly asymmetrical as plus-strand RNAs are produced in fast excess.
A hallmark of nidovirus transcription is the production of a 3′-coterminal nested set of sg mRNAs. The sg mRNAs of corona-, arteri- and bafiniviruses are chimeric, that is, comprised of sequences that are non-contiguous in the viral genome. Each carries a short 5′ leader sequence of 55–92, 170–210 nt, and 42 nucleotides, respectively, which is identical to the 5′ end of the viral genome. It was established early on that leader and “body” sequences are not joined through splicing, but via a process of discontinuous RNA synthesis. A key observation was the presence of mirror-copy nested sets of sg minus-strand RNAs in corona- and arterivirus-infected cells. Combined experimental evidence from biochemical and reverse genetics analyses indicates that these sg minus-strand RNAs are in fact the templates for sg mRNA synthesis. Replicative intermediates (RI)/replicative forms with sizes corresponding to the different sg mRNAs were shown to be actively involved in transcription. According to the prevailing 3′-discontinuous extension model, the discontinuous step occurs during the production of sg minus-strand RNAs and entails attenuation of RNA synthesis at the TRSs, followed by a similarity-assisted copy choice RNA recombination event. In corona-, arteri- and bafiniviruses, a TRS is present immediately downstream of the genomic leader sequence. It is believed that, during minus-strand RNA synthesis, the replicase complex upon encounter of an internal TRS dissociates from the template and is transferred to the 5′ end of the genome, guided by sequence complementarity between the anti-TRS on the nascent strand and the genomic TRS. Reinitiation and completion of RNA synthesis would then result in a chimeric minus-strand that in turn would serve as a template for uninterrupted (continuous) synthesis of 5′ leader-containing sg mRNAs.
Discontinuous sg RNA synthesis is not a trait of all nidoviruses. Ronivirus sg mRNAs lack a common 5′ leader and thus apparently arise from non-discontinuous RNA synthesis. Toroviruses employ a mixed transcription strategy; of the four sg RNAs, only RNA 2 carries a 15–18 nt 5′ leader derived from the 5′ end of the genome, whereas the others do not. It is likely that sg mRNAs are transcribed from sg minus-strand templates also in toro- and in roniviruses. Here, the conserved sequence elements (TPs) preceding the 3′-proximal genes might serve dual roles as signals for premature termination of minus-strand synthesis and as promoters for plus-strand production. The torovirus S gene, expressed from mRNA 2, lacks a TP. Apparently, transcription-competent minus-strand sg RNAs are produced by inclusion of a complementary copy of the 5′-terminal genomic TP via a similarity-assisted RNA recombination process analogous to that seen in corona- and arteriviruses.
Antigenic properties
In coronaviruses, the S protein is an important target for T cell responses and is the major inducer of virus-neutralizing antibodies, which are elicited by epitopes located mostly in the N-terminal half of the molecule. The surface-exposed N-terminus of the M protein induces antibodies that neutralize virus infectivity in the presence of complement. The N protein is a dominant antigen during the natural infection and, like the S protein, might evoke protective T cell responses. HE induces antibodies that prevent binding to O-acetylated sialic acids or inhibit sialate-O-acetylesterase activity. The ectodomains of the S and HE proteins are highly variable, suggestive of extensive antigenic drift. In addition, there are several examples of intergenotypic exchange of coding sequences of S (for Avian coronavirus, Murine coronavirus and for the feline and canine coronaviruses belonging to Alphacoronavirus 1) and HE (Murine coronavirus) ectodomains through homologous RNA recombination, consistent with the occurrence of antigenic shifts.
All toroviruses described so far are serologically related. During natural infection, antibodies are raised against each of the four structural proteins (S, HE, M and N). The spike (S) protein induces virus-neutralizing antibodies; sera from BToV- or PToV-infected animals cross-neutralize EToV. Comparative sequence analysis of bovine and porcine torovirus field variants revealed several instances in which coding sequences for the HE ectodomain had been exchanged through intergenotypic homologous RNA recombination. Bovine torovirus variants currently prevalent in the field (genotypes II and III) have apparently arisen from a recombination event during which the ancestral BToV (genotype I) swapped its N gene for that of porcine torovirus.
Antibodies against the known arteriviruses (EAV, LDV, PRRSV, SHFV) do not cross-react and there is considerable antigenic variation among different strains of EAV, LDV and PRRSV. Major glycoprotein GP5 (designated GP7 in SHFV) is the main determinant of virus-neutralization. In some arteriviruses (PRRSV type I), minor glycoprotein GP4 also induces neutralizing antibodies. A number of arterivirus proteins have been reported to evoke T cell responses, including GP5 and M.
At present, there are no data available about the antigenic properties of bafinivirus proteins or about the innate defense responses mounted against roniviruses in their invertebrate hosts. Serological interfamily or intergenus cross-reactivity has not been demonstrated.
Biological properties
Coronaviruses infect birds and mammals, including humans, livestock and companion animals. Bats are believed to play a pivotal role in CoV ecology and evolution as they appear to harbor an exceptionally wide diversity of CoVs. It has even been proposed that bats may be the original hosts from which many if not all alpha- and betacoronavirus lineages are derived.
CoVs predominantly target the epithelia and, consequently, infections are mostly associated with respiratory and gastrointestinal disease. Biological vectors are not known. Depending on the virus species, coronaviruses are transmitted via aerosols, fomites or the fecal–oral route. In many instances a persistent chronic infection develops with prolonged shedding of virus from the enteric tract. Coronavirus infections are often mild. However, in 2002–2003, a novel coronavirus, SARS-CoV, caused an epidemic in human populations of a severe pulmonary disease with a mortality rate of 10%. For other CoVs, hepatitis and infection of the central nervous system (MHV), heart and eye (RbCoV) have been described. Variants of Alphacoronavirus 1 (feline, canine and ferret coronaviruses) may infect cells of the monocyte/macrophage lineage and cause fatal systemic infections characterized by wide-spread granulomatous lesions in multiple organs.
Toroviruses infect ungulates: horses (EToV, Berne virus), bovines (BToV, Breda virus) and swine (PToV). Humans (HToV) and probably carnivores (mustellids) have also been proposed as hosts for toroviruses. Transmission is probably by the fecal–oral route.
The bafinivirus white bream virus is the only known teleost nidovirus and so far was isolated from one species of fresh water fish (Blicca bjoerkna L.). At present no further information is available on its ecology, biology and pathogenic properties.
Arteriviruses infect horses (EAV), mice (LDV), monkeys (SHFV) and swine (PRRSV). Primary host cells for all arteriviruses are macrophages. EAV causes inflammation of small arteries and EAV infection can lead to a wide range of clinical manifestations. A fatal outcome of the disease has been reported in both natural and experimental infections, but most natural infections are either mild or subclinical. In pregnant animals, arteriviruses can cause abortions (PRRSV and EAV) or in utero fetal death (PRRSV). Persistent infections – lifelong in the case of LDV – are frequently established. Virus may be shed in saliva and respiratory secretions, feces, urine and milk. Persistently-infected males may shed virus in the semen (EAV, PRRSV). Spread is in general horizontal, via direct contact, aerogenic, fecal–oral and/or venereal transmission routes.
Roniviruses are the only known invertebrate nidoviruses and have been detected exclusively in crustaceans. The black tiger prawn (Penaeus monodon) appears to be the natural host of YHV and gill-associated virus (GAV), but other prawn species are susceptible to experimental infection. Infections may be chronic or acute and transmission can occur horizontally and vertically. During acute infections, mortality is usually high and virus occurs in most tissues of ectodermal and mesodermal origin, and particularly in the “Oka” or lymphoid organ. Necrotic cells display intensely basophilic cytoplasmic inclusions. The geographic range of infection encompasses the natural Indo-Pacific distribution of P. monodon, in which the prevalence of subclinical infection is commonly high, and there is recent evidence of infection occurring in shrimp species farmed in the Americas.
Phylogenetic relationships within the order
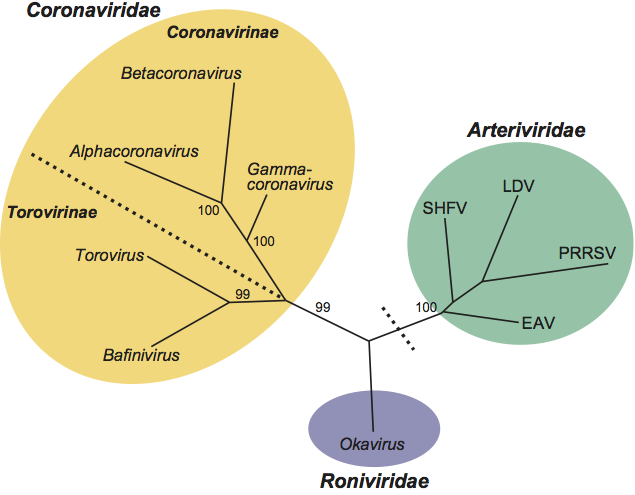
In rooted and unrooted phylogenetic trees constructed for the main replicative enzymes, members of the families Corona-, Arteri- and Roniviridae consistently form distinct, well-separated monophyletic clusters. Viruses in the subfamily Torovirinae (genera Bafini- and Torovirus) are phylogenetically more related to each other than to those in the subfamily Coronavirinae. The evolutionary relationships between nidovirus (sub)families and genera are illustrated in Figure 4.
Figure 4 Nidovirus phylogeny. The evolutionary relationships between the five major nidovirus lineages are depicted by an unrooted maximum parsimonious tree, inferred by using multiple nucleotide sequence alignments of the RdRp-Hel region of representative members of nidovirus (sub)families and genera. For the main bifurcations, support from 100 bootstraps is given. (Sub)families and genera are highlighted and/or labeled. For arteriviruses, the four main clusters, prototyped by EAV, SHFV, LDV and PRRSV (only one of two currently recognized genotypes shown), are indicated. The divisions between large (Coronaviridae, Roniviridae) and small (Arteriviridae) nidoviruses and the one between Corona- and Torovirinae are indicated by black dotted lines.
(Modified from Gorbalenya, A.E. (2008). Genomics and evolution of the Nidovirales. In: Perlman, Gallagher and Snijder (Eds.), Nidovirales. ASM Press, Washington DC, pp.15–28.)
Similarity with other taxa
Nidoviruses can be uniquely distinguished from other RNA viruses on the basis of their replicase polyproteins that comprise a number of characteristic domains arranged in a conserved order. Key diagnostic molecular markers are:
- An ORF1a-encoded protease with a chymotrypsin-like fold and substrate specificity resembling that of picornavirus 3C protease (3C-like protease, also called main protease, Mpro), flanked by two hydrophobic transmembrane domains (tm-Mpro-tm).
- An ORF1b-encoded putative multinuclear Zn-finger-like domain associated with a nucleoside triphosphate (NTP)-binding/5′-to-3′-helicase domain (Zn-HEL).
- The replicase gene constellation separated by a ribosomal frameshifting signal (fs): tm-Mpro-tm_fs_RdRp_Zn-HEL.
Homologs of several (putative) enzymes encoded by viruses of the order Nidovirales have been found in non-nidoviruses. The proteolytic enzymes and RdRps cluster together with homologs of viruses of the “Picornavirus-like” supergroup, and RdRps also with homologs in double stranded RNA Birnaviridae family members and a subset of members of the family Tetraviridae. The nidovirus helicase and ADRP have counterparts in viruses of the “Alphavirus-like” supergroup. The organization of the replicase ORFs, including the Mpro_FS_RdRp constellation, is also conserved in the family Astroviridae and in some viruses in the Sobemo-like supergroup. Parallels in the genome organization and expression strategy are also evident between members of the order Nidovirales and the family Closteroviridae.
Derivation of names
Nido: from Latin nidus, “nest”, refers to the synthesis of a 3′-coterminal, nested set of mRNAs, hallmark of nidovirus transcription.
Arteri: from equine arteritis, the disease caused by the reference virus.
Corona: from Latin corona, “halo”; refers to the characteristic appearance of surface projections that create an image reminiscent of the solar corona.
Toro: from Latin torus, a term used in architecture for the convex molding at the base of a column and in geometry for a three-dimensional structure in the shape of a hollow donut; refers to the nucleocapsid morphology in a subset of particles.
Bafini: from bacilliform fish nidoviruses, refers to the virion morphology and host tropism.
Roni: from rod-shaped nidoviruses, refers to the virion morphology.
Note added in proof
During the completion of this manuscript, two papers appeared reporting the discovery of insect nidoviruses. These mosquito-associated nidoviruses are likely representatives of a novel family within the order Nidovirales.
Further reading
Journals and books
Bárcena et al., 2009 M. Bárcena, G.T. Oostergetel, W. Bartelink, F.G. Faas, A. Verkleij, P.J. Rottier, A.J. Koster, B.J. Bosch, Cryo-electron tomography of mouse hepatitis virus: insights into the structure of the coronavirion. Proc. Natl Acad. Sci., U S A. 106 (2009) 582–587.
Enjuanes et al., 2006 L. Enjuanes, F. Almazan, I. Sola, S. Zuñiga, Biochemical aspects of coronavirus replication and virus host-interaction. Ann. Rev. Microbiol. 60 (2006) 211–230.
Gorbalenya et al., 2006 A.E. Gorbalenya, L. Enjuanes, J. Ziebuhr, E.J. Snijder, Nidovirales: evolving the largest RNA virus genome. Virus Res. 117 (2006) 17–37.
Nga et al., 2011 P.T. Nga, M.D.C. Parquet, C. Lauber, M. Parida, T. Nabeshima, F. Yu, N.T. Thuy, S. Inoue, T. Ito, K. Okamoto, A. Ichinose, E.J. Snijder, K. Morita, A.E. Gorbalenya, Discovery of the first insect nidovirus, a missing evolutionary link in the emergence of the largest RNA virus genomes. PLoS Pathogens. (2011) 7.
Pasternak et al., 2006 A.O. Pasternak, W.J. Spaan, E.J. Snijder, Nidovirus transcription: how to make sense …?. J. Gen. Virol. 87 (2006) 1403–1421.
Perlman et al., 2008 S. Perlman, T. Gallagher, E.J. Snijder, Nidoviruses. In: S. Perlman, T. Gallagher, E.J. Snijder, Nidoviruses. ASM Press, Washington, DC2008.
Perlman and Netland, 2009 S. Perlman, J. Netland, Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 7 (2009) 439–450.
Sawicki et al., 2007 S.G. Sawicki, D.L. Sawicki, S.G. Siddell, A contemporary view of coronavirus transcription. J. Virol. 81 (2007) 20–29.
Schütze et al., 2006 H. Schütze, R. Ulferts, B. Schelle, S. Bayer, H. Granzow, B. Hoffmann, T.C. Mettenleiter, J. Ziebuhr, Characterization of White bream virus reveals a novel genetic cluster of nidoviruses. J. Virol. 80 (2006) 11598–11609.
Spilman et al., 2009 M.S. Spilman, C. Welbon, E. Nelson, T. Dokland, Cryo-electron tomography of porcine reproductive and respiratory syndrome virus: organization of the nucleocapsid. J. Gen. Virol. 90 (2009) 527–535.
Thiel, 2007 V. Thiel, Coronaviruses: Molecular and Cellular Biology. Caister Academic Press, Norfolk2007.
Zirkel et al., 2011 F. Zirkel, A. Kurth, P-L. Quan, T. Briese, H. Ellerbrok, G. Pauli, F.H. Leendertz, W.I. Lipkin, J. Ziebuhr, C. Drosten, S. Junglen, . An insect nidovirus emerging from a primary tropical rainforest. mBio. 2 (2011) E00077–11.
Websites
VIPR Virus Pathogen Resource: http://www.viprbrc.org/brc/
Contributed by
de Groot, R.J., Cowley, J.A, Enjuanes, L., Faaberg, K.S., Perlman, S., Rottier, P.J.M., Snijder, E.J., Ziebuhr, J. and Gorbalenya, A.E.
