Realm: Duplodnaviria
Evelien M. Adriaenssens, Ryan Cook, Valerian Dolja, Eugene V. Koonin, Mart Krupovic, Jens H. Kuhn, Cédric Lood, Alejandro Reyes Muñoz, Dann Turner, Arvind Varsani, Paola K. Vaz, Thomas Waltzek, Yuri I. Wolf, Natalia Yutin, F. Murilo Zerbini
The citation for this ICTV Report chapter is the summary to be published as Adriaenssens et al., (2025):
ICTV Virus Taxonomy Profile: Duplodnaviria 2025, Journal of General Virology, (in press)
Corresponding author: Evelien M. Adriaenssens ([email protected])
Edited by: Simon Roux and Hanna Oksanen
Posted: July 2025
Summary
The realm Duplodnaviria was established in 2020 (Master Species List #35) for viruses with double-stranded DNA (dsDNA) genomes and having a major capsid protein (MCP) containing the HK97-fold domain. Moreover, duplodnavirians share a distinct morphogenetic module that consists of four hallmark genes encoding the HK97-fold MCP, a genome packaging ATPase-nuclease (large terminase subunit) that is distinct from the functional counterpart of varidnavirians, a portal protein, and a capsid maturation protease (Rixon and Schmid 2014, Hawkins et al., 2024, Koonin et al., 2024). The hosts of duplodnavirians include bacteria and archaea (phylum Uroviricota), as well as eukaryotes (phylum Peploviricota) (Table 1 Duplodnaviria).
Table 1 Duplodnaviria. Characteristics of members of the realm Duplodnaviria
| Characteristic | Description |
| Example 1 | pseudorabies virus (JF797218), species Varicellovirus suidalpha1 |
| Example 2 | Escherichia phage T4 (AF158101), species Tequatrovirus T4 |
| Example 3 | Haloferax tailed virus 1 (MG550112), species Retbasiphovirus hantatum |
| Virion | Icosahedral capsids, with or without an envelope, with (bacterial and archaeal viruses) or without (eukaryotic viruses) helical tails |
| Genome | A single linear segment of dsDNA, length range of 11.6–660 kbp. Reiterated sequences are common. Terminase-type DNA packaging. Modular and scattered gene organization
|
| Replication | Rolling concatemeric, replicative transposition, or protein-primed DNA replication using virus or host encoded enzymes. DNA-templated transcription by host or virus-encoded RNA polymerase, may be temporally co-ordinated. |
| Translation | Some mRNAs are spliced. |
| Host range | Bacteria, archaea, eukaryotes |
| Taxonomy | The realm (MSL #40) includes 1 kingdom, 2 phyla, 2 classes, 12 orders, 108 families, 135 subfamilies, 1703 genera, 5932 species (of which 2401 species are unassigned to a family) |
Virion
Morphology
Particles of viruses of the class Herviviricetes (phylum Peploviricota) are 150–200 nm in diameter. Particles are described as pleomorphic, mostly spherical, and have a glycoprotein-containing envelope that encloses a tegument and an icosahedral capsid (Figure 1 Duplodnaviria).
Particles of viruses of the class Caudoviricetes (phylum Uroviricota) are described as head-tailed, or predicted head-tailed, and do not have an envelope (Baquero et al., 2020, Dion et al., 2020). The head (capsid) is icosahedral and may be isometric or prolate. The head diameter is generally 40–200 nm. The length of the tail is variable, typically 10–350 nm, and up to about 800 nm in some bacterial viruses (Minakhin et al., 2008). The tail extends from one vertex (Figure 2 Duplodnaviria). Three major morphotypes are recognised based on the tail structure, but these are no longer considered taxonomic features: viruses of the myo morphotype have long, contractile tails; viruses of the sipho morphotype have long, non-contractile tails; and viruses of the podo morphotype have short, non-contractile tails (Ackermann 1998, Ackermann 2001).
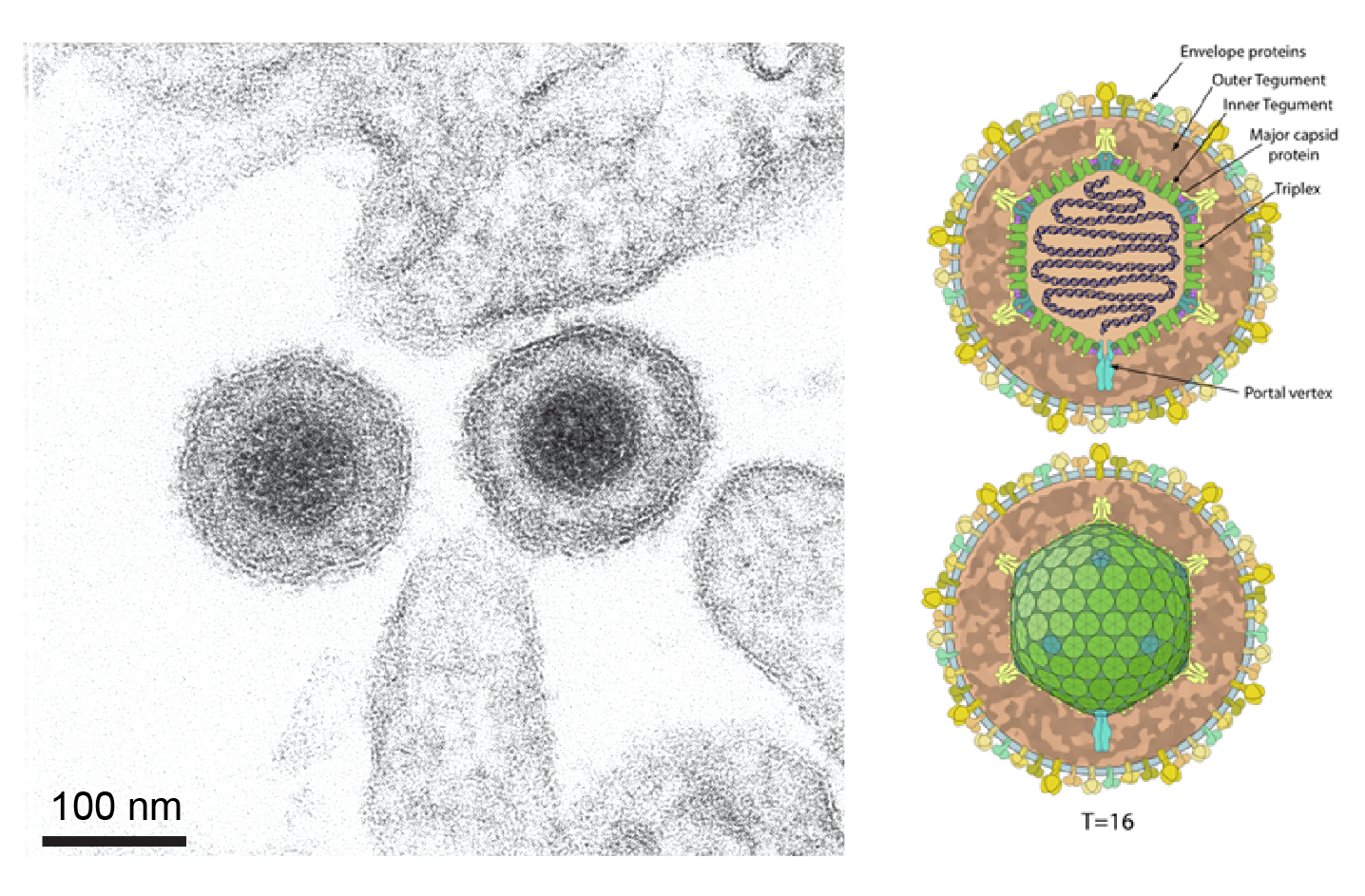 |
| Figure 1 Duplodnaviria. Herviricete particle example. (Left) Electron micrograph of extracellular pseudorabies virus (family Orthoherpesviridae, species Varicellovirus suidalpha1). Scale bar = 100 nm. The image is taken from Figure 1 in (Klupp and Mettenleiter 2023) under a Creative Commons Attribution 4.0 International License (Right) Schematic of an orthoherpesvirid virus particle. The image is taken from ViralZone (viralzone.expasy.org), under a Creative Commons Attribution 4.0 International License (credit: SwissBioPics) |
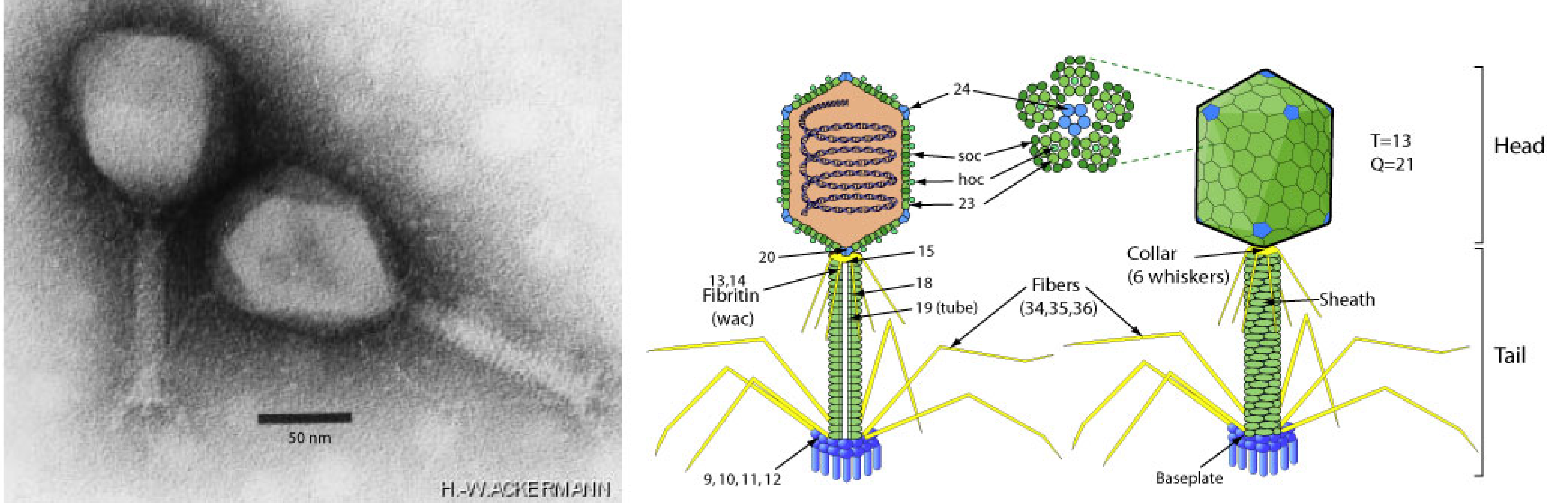 |
| Figure 2 Duplodnaviria. Caudoviricete particle example. (Left) Electron micrograph of Escherichia phage T4 also known as Enterobacteria phage T4 (family Straboviridae, species Tequatrovirus T4). Scale bar = 50 nm. Micrograph taken by Hans-Wolfgang Ackermann. (Right) Schematic of a strabovirid virus particle. Both images from ViralZone (viralzone.expasy.org) under a Creative Commons Attribution 4.0 International License (credit: SwissBioPics) |
Nucleic acid
All duplodnavirians have linear dsDNA genomes when packaged in the particle. Herviviricete genomes (Figure 3 Duplodnaviria) are 108.4–322.3 kbp in length. Caudoviricete genomes range from 11.6 to more than 500 kbp in length. In most duplodnavirians, the genomes have defined termini, with reiterated sequences (direct or inverted repeats), cohesive overhangs (3′ or 5′) or circularly permuted genome architectures that reflect the wide range of replication and packaging mechanisms found in viruses of the taxon (Garneau et al., 2017, Gatherer et al., 2021, Gulyaeva et al., 2022). In some caudoviricetes, the genomes contain covalently linked terminal proteins (Redrejo-Rodríguez and Salas 2014) or covalently linked hairpins (Ravin 2015). Duplodnavirians use a terminase-type DNA packaging system.
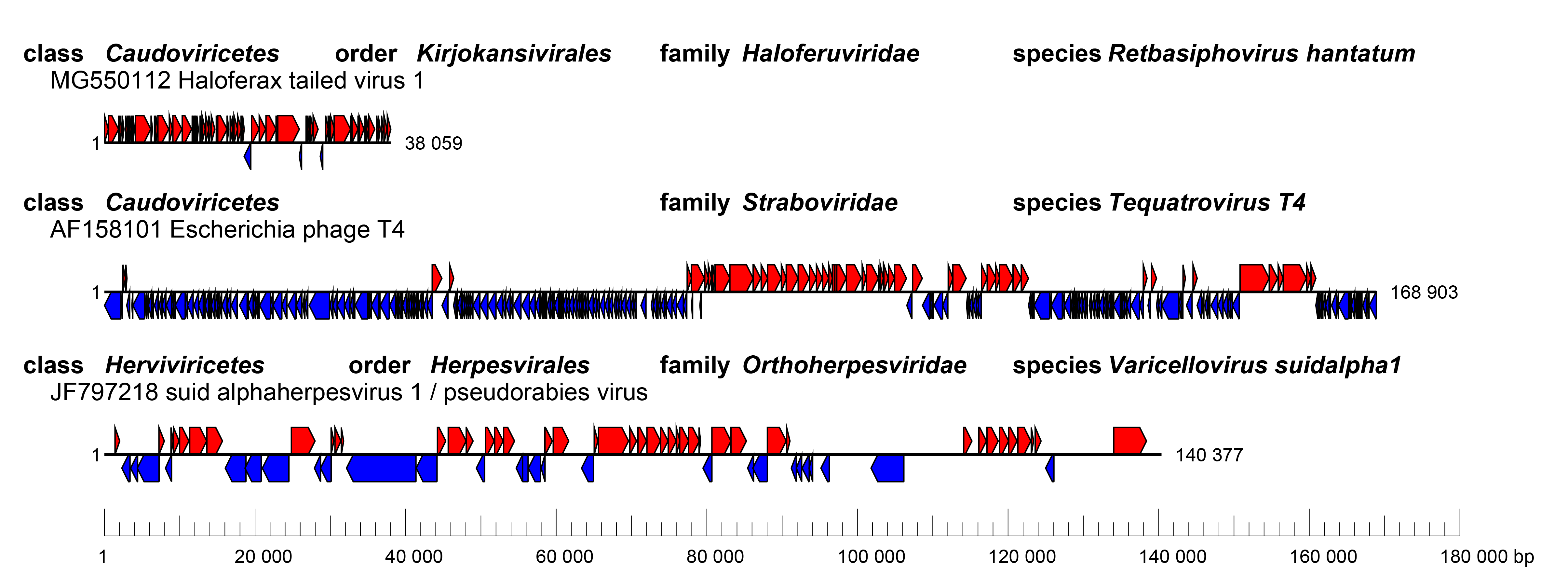 |
| Figure 3 Duplodnaviria. Genome organization of three example viruses classified in the realm Duplodnaviria. (Top) Haloferax tailed virus 1 (MG550112), (Middle) Escherichia phage T4 (AF158101), members of the class Caudoviricetes, (Bottom) pseudorabies virus (JF797218), a member of the class Herviviricetes.. Boxes indicate coding sequences as annotated in the respective GenBank records, colored according to the coding strand. |
Proteins
The protein composition of the mature herpesviral particle varies greatly among viruses of different families, with, for example, more than 30 different protein species in herpes simplex virus 1 (HSV1, family Orthoherpesviridae, species Simplexvirus humanalpha1) virions. The mature HSV1 capsid is composed of four major and several minor proteins, and the tegument contains at least 15 proteins (Dai and Zhou 2018). The viral envelope contains at least ten integral membrane proteins, including the membrane fusion protein. In the known herpesvirals, the HK97-fold is found in the floor domain of the major capsid protein (Rixon and Schmid 2014).
The caudoviricete particle is exemplified by Escherichia phage T4 (order Pantevenvirales, family Straboviridae, species, Tequatrovirus T4). The T4 virion, with a prolate icosahedral capsid (head) and a contractile tail, is constructed from about 40 proteins, including the HK97-fold containing major capsid protein, several minor capsid proteins, including the portal protein, and multiple tail proteins (Yap and Rossmann 2014). The packaging motor complex is assembled at the portal vertex and connects to the tail that terminates with a hexagonal baseplate attached to tail fibers. The T4 head, tail, and fibers assemble by separate pathways and join to form the virion (Rao et al., 2023). Tail proteins are not conserved across viruses of the class.
The terminase-type DNA packaging motor of duplodnavirians is typically comprised of three key components: a portal protein, a motor protein (including both ATPase and nuclease domains), and an initiator of the DNA packaging. The motor and initiator proteins are referred to as the large and small subunits of the terminase, respectively (Bohmer et al., 2023, Rao et al., 2023).
Lipids
Only herpesviral particles have lipid envelopes and they are not well characterized. The HSV1 envelope is enriched in sphingomyelin and phosphatidylserine (van Genderen et al., 1994). During human cytomegalovirus (HCMV, family Orthoherpesviridae, species Cytomegalovirus humanbeta5) infection, there is increased production of very long chain fatty acids, and saturated forms of these lipids are incorporated selectively into the virion envelope (Koyuncu et al., 2013).
Carbohydrates
Herpesviral virion envelopes contain multiple proteins that bear N-linked and O-linked glycans. Mature, cell-free virions contain complex glycans, whereas intracellular virions can have N-linked glycans of the immature high-mannose type (Johnson and Spear 1983). O-linked glycosylation sites have been mapped globally for several orthoherpesvirids by mass spectrometric methods (Bagdonaite et al., 2016). O-linked glycosylation of the capsid proteins has been also reported for certain caudoviricetes infecting Mycobacteria (Freeman et al., 2023).
Genome organization and replication
The genome organization of duplodnavirians includes modular arrangements, i.e., clustering of genes with associated functions (especially in caudoviricetes with short genomes) with conserved, essential genes typically localised internally/centrally in the genome (Figure 4 Duplodnaviria), but can also occur in scattered gene arrangements (for example in members of the order Pantevenvirales). Many caudoviricetes also encompass arrays of genes involved in virus-host interactions, such as inhibitors of host defence systems. Often, but not always, modular arrangement correlates with temporally co-ordinated gene expression in a kinetic cascade such that early expressed and late expressed genes are partitioned in the genome.
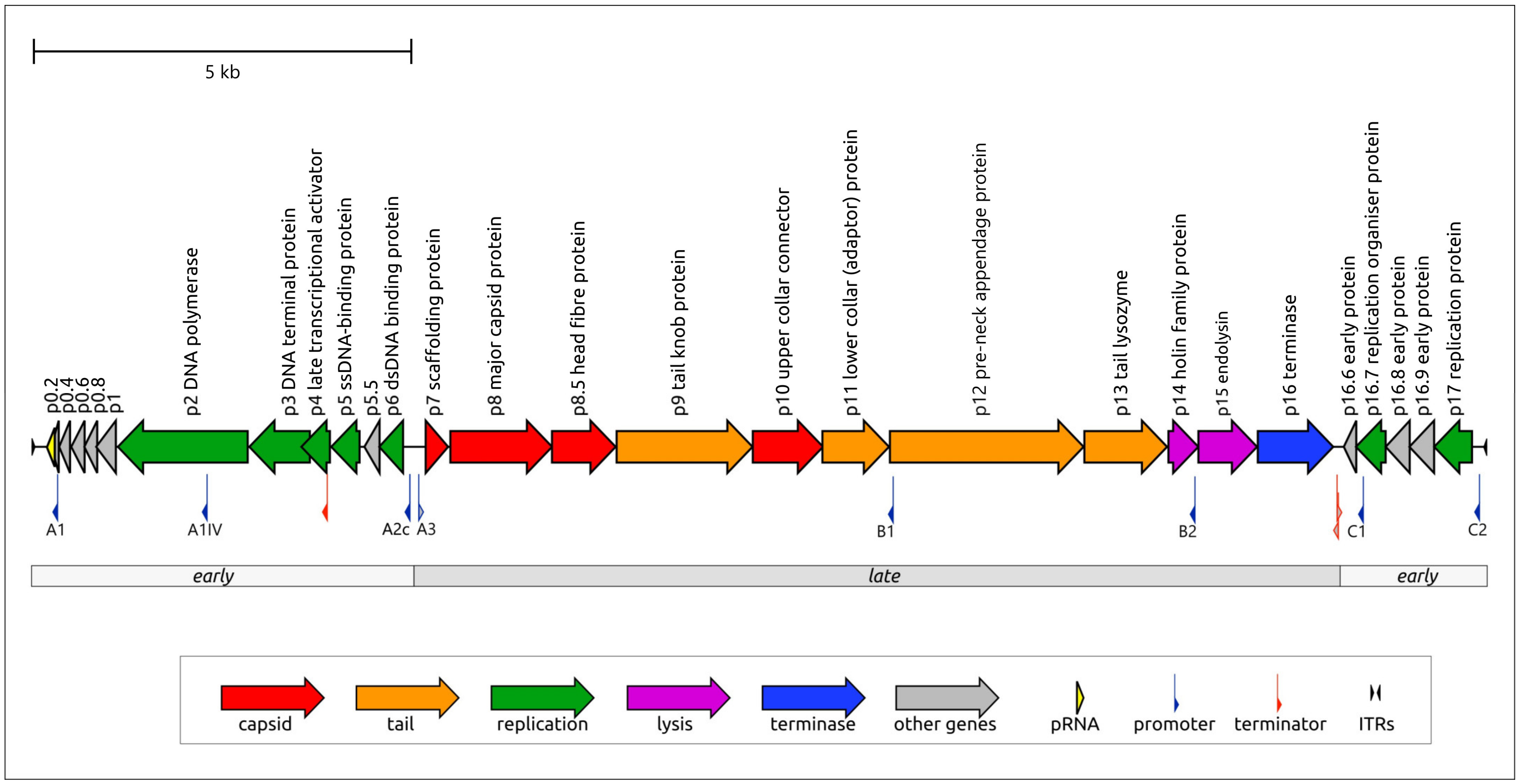 |
| Figure 4 Duplodnaviria. The modular organization of the Bacillus phage phi29 (family Salasmaviridae, species Salasvirus phi29) genome (GenBank EU771092). Arrows indicate the direction of transcription for each gene. The gene products are shown in accordance with experimentally assigned functions. The genome length is 19.2 kbp. The figure corresponds to Figure 1 in (Evseev et al., 2024), under a Creative Commons Attribution 4.0 International License |
Duplodnavirians use either host cell or a virus-encoded DNA-directed RNA polymerase for transcription of viral genes. In eukaryotic cells, DNA-directed RNA polymerase II (Pol II) transcribes herpesviral genes producing capped, polyadenylated mRNAs, which generally encode single proteins and are not spliced, with the exception of the large terminase subunit and the catalytic subunit of the DNA polymerase in orthoherpesvirids. The lytic replication cycle of orthoherpesvirids in permissive cells is a classic example of temporally co-ordinated gene expression with the consecutive expression of immediate early, early and late proteins (Gatherer et al., 2021).
Duplodnavirians use diverse mechanisms for genome replication. Typically, duplodnavirians with larger genomes encode their own DNA polymerases (Kazlauskas et al., 2016). Herpesvirals uniformly encode family B DNA polymerases along with several other replication proteins, suggesting semiautonomous genome replication (Kazlauskas et al., 2016). Caudoviricetes can encode DNA polymerases of families A, B (both RNA- and protein-primed) and C (Yutin et al., 2024). The replication protein complements of caudoviricetes vary from minimalistic to nearly complete replisomes. Some caudoviricetes with larger genomes encode homologs of most cellular DNA replication proteins (Philosof et al., 2017), whereas viruses with smaller genomes encode only the key replisome components which enable recruitment of the host replication machinery. Another canonical mechanism for genome replication is found in a group of temperate phages known as transposable phage (e.g., the coliphage Mu, species Muvirus mu). Transposable phages undergo replicative transposition, driven by the transposome complex (Montaño et al., 2012), during which the viral genome is copied at various sites of the bacterial chromosome before initiating transcription of the structural proteins and subsequent packaging (Faelen et al., 1978).
Biology
Herpesvirals infect both vertebrates and invertebrates. Orthoherpesvirids and alloherpesvirids infect a broad range of vertebrate hosts, extending from fish and amphibians to reptiles/birds, and mammals. Malacoherpesvirids infect invertebrates (molluscs). Typically, the natural host range of individual viruses is restricted. Many orthoherpesvirids are highly adapted to their hosts, so that severe infection is usually observed only in the foetus, the very young, the immunocompromised or following infection of an alternative host. This conditional pathology reflects the ability of vertebrate herpesviruses to establish life-long latent infection in neurons, haematopoietic cells, or mononuclear cells.
Orthoherpesvirid infection elicits a wide variety of innate and adaptive immune responses, and many structural and non-structural viral proteins are antigenic, with individual proteins harbouring multiple epitopes. Some envelope glycoproteins are targets for neutralizing antibodies. Cell-mediated immune responses, rather than neutralising antibodies, provide a stronger level of protection from recurrences and reinfection (Truong et al., 2019).
Caudoviricetes can infect a wide variety of bacterial and archaeal hosts, and are highly abundant in many environments. Caudoviricetes are the most commonly recovered viruses using shotgun metagenomics approaches in most mesophilic, extreme and host-associated environments and make up the largest fraction of uncultivated virus sequence databases (Camargo et al., 2023). Integrated caudoviricetes (prophages/proviruses) have been identified in the genome of most phyla of bacteria and archaea (Liu et al., 2021, López-Leal et al., 2022, Medvedeva et al., 2022, Medvedeva et al., 2023, Sharma et al., 2023, Dieppa-Colón et al., 2025).
Bacteria-infecting caudoviricetes with strictly lytic lifestyles, i.e. virulent phages, have been used successfully in phage therapy, in compassionate use cases (Pirnay et al., 2024) or as regulated therapy as in recognised phage therapy centres such as the Eliava Institute in Georgia, the Hirszfeld Institute in Poland and in various centres in Russia (Międzybrodzki et al., 2021).
Archaeal and bacterial caudoviricetes are thought to have diverged from their common ancestor concomitantly with the divergence of bacteria and archaea from the last universal cellular ancestor (Krupovic et al., 2020). Archaeal and bacterial caudoviricetes share the same general principle of virion organization and maturation (Baquero et al., 2020) as well as similar strategies of interaction with the hosts (e.g., can be either virulent or temperate).
Derivation of names
Autographivirales: from the Ancient Greek words αὐτός (autós), meaning “self” and γράφειν (gráphein), meaning “write” referencing the presence of a single subunit DNA-dependent RNA polymerase; the suffix -virales for order taxa
Caudoviricetes: from the Latin caudo, meaning tail; referring to the virion morphology of viruses in the taxon; the suffix -viricetes for class taxa
Crassvirales: from the prototypical uncultured virus, crAssphage, a name derived from the software tool crAss for cross-assembly analysis; the suffix -virales for order taxa
Duplodnaviria: from the Latin dūplō, meaning double and DNA, referring to the fact that all founding members of the realm have double-stranded dsDNA genomes; the suffix -viria for realm taxa
Grandevirales: from the word grande, meaning large or great in Latin and other European languages; the suffix -virales for order taxa.
Herpesvirales: from the Ancient Greek ἕρπω (hérpō) meaning "to creep", referring to spreading blisters, with the addition of the consonant s; the suffix -virales for order taxa
Herviviricetes: from herpesvirus; referring to the herpesviruses, members of the class; the suffix -viricetes for class taxa
Heunggongvirae: from the Cantonese 香港 (Hēunggóng), meaning (and approximately pronounced) Hong Kong— referring to Escherichia coli phage HK97, the founding member of the HK97 (Hong Kong 97)-fold major capsid protein viruses in this taxon; the suffix -virae for kingdom taxa
Juravirales: from the Lithuanian jūra, meaning "sea"; referring to the marine archaeal viruses in the taxon; the suffix -virales for order taxa
Kirjokansivirales: from the Finnish kirjo, meaning "versatility" or "variety", and from the Finnish kansi, meaning "cover", a magical object in Finnish mythology which brought riches and happiness including salt to its holder, referring to the haloarchaeal hosts of viruses in the order; the suffix -virales for order taxa
Magrovirales: from Marine Group II Euryarchaeota (now Methanobacteriati), the historical name of Candidatus Poseidoniales archaea; the suffix -virales for order taxa
Methanobavirales: from Methanobacteriales, referring to the host of the viruses; the suffix -virales for order taxa
Nakonvirales: from Nakon, the most powerful god of war in Mayan mythology; the suffix -virales for order taxa
Pantevenvirales: from the Greek word πᾶν (pân) in its meaning of “all-inclusive” in combination with T-even, the description that was used for bacterial viruses related to the Escherichia coli phages T2, T4 and T6, founding members of the taxon; the suffix -virales for order taxa
Peploviricota: from the name of a higher-rank taxon proposed by Lwoff and Tournier in 1966 (“Peplovirales”; from the Greek πέπλος (peplos)), meaning garment, referencing the unique tegument of herpesvirals; the suffix -viricota for phylum taxa
Thumleimavirales: from the Meitei word ꯊꯨꯝꯂꯩꯃ (Thumleima), the goddess and the female personification of salt, and natural salt brines in Meitei mythology, referring to the haloarchaeal hosts of viruses in the order; the suffix -virales for order taxa
Uroviricota: from the name of a higher-rank taxon for tailed phages proposed by Lwoff and Tournier in 1966 (“Urovirales”; from the Greek οὐρά (ourá/uros)), meaning tail; the suffix -viricota for phylum taxa
Demarcation criteria for taxa within the realm
Duplodnaviria: a virus is a member of this realm if it has a dsDNA genome encoding a major capsid protein containing the HK97 fold. Viruses of this realm also share portal protein and the terminase complex, which is unrelated to the genome packaging ATPase conserved in the majority of dsDNA viruses with a vertical jelly-roll major capsid protein.
Peploviricota: a Duplodnaviria member is a member of the included phylum Peploviricota if it infects eukaryotes, but not prokaryotes.
Uroviricota: a Duplodnaviria member is a member of the included phylum Uroviricota if it infects prokaryotes, but not eukaryotes.
If a principal rank taxon includes only a single lower-ranked taxon, then the definition of the lower-ranked taxon is, for now, identical to the definition of the higher-ranked taxon. Thus:
Duplodnaviria ≡ Heunggongviraea
Peploviricota ≡ Herviviricetes ≡ Herpesvirales
Uroviricota ≡ Caudoviricetes
The coherence of the order Herpesvirales rests on virion morphology, where the large linear dsDNA genome is contained within a T=16 icosahedral capsid, which is surrounded by a proteinaceous tegument and then by a lipid envelope containing a variety of membrane-associated proteins. Further demarcation (of lower rank taxa) relies primarily on phylogenetic comparisons of conserved protein coding genes, such as the viral DNA-directed DNA polymerase.
The class Caudoviricetes, originally established as the order “Caudovirales” (based upon a head-tail virion morphology), includes tailed viruses infecting bacteria and archaea. Autographivirales, Crassvirales, Grandevirales, Pantevenvirales are orders comprising bacteria-infecting viruses. Members of these taxa are identified by conserved genome architecture, the partial conservation of gene order and monophyly of conserved protein-coding genes. The Juravirales, Kirjokansivirales, Magrovirales, Methanobavirales, Nakonvirales, and Thumleimavirales are orders of tailed viruses that infect archaea. Orders in the class Caudoviricetes are delineated genotypically by a variety of bioinformatic analyses, including all-versus-all genomic similarity matrices, heatmaps of fractions of orthologous genes, phylogenomic trees of global proteomes, phylogenetic analysis of conserved genes, and network analysis of shared protein clusters.
The demarcation criteria that delineate families, subfamilies, genera, subgenera and species can be found in the online report family chapters, or relevant taxonomy proposals.
Taxonomic relationships within the realm
The taxonomic relationships at the realm rank down to the class rank cannot be captured with any of the currently available phylogenetic methods, and the relationships are represented as a cladogram based on the demarcation criteria (Figure 5 Duplodnaviria). Although all duplodnavirians share conserved hallmark genes, the sequence divergence across the realm is too high to allow construction of reliable multiple sequence alignments and phylogenies.
Proteome-based computational methods are currently used to delineate orders and families within the class Caudoviricetes (Figure 6 Duplodnaviria).
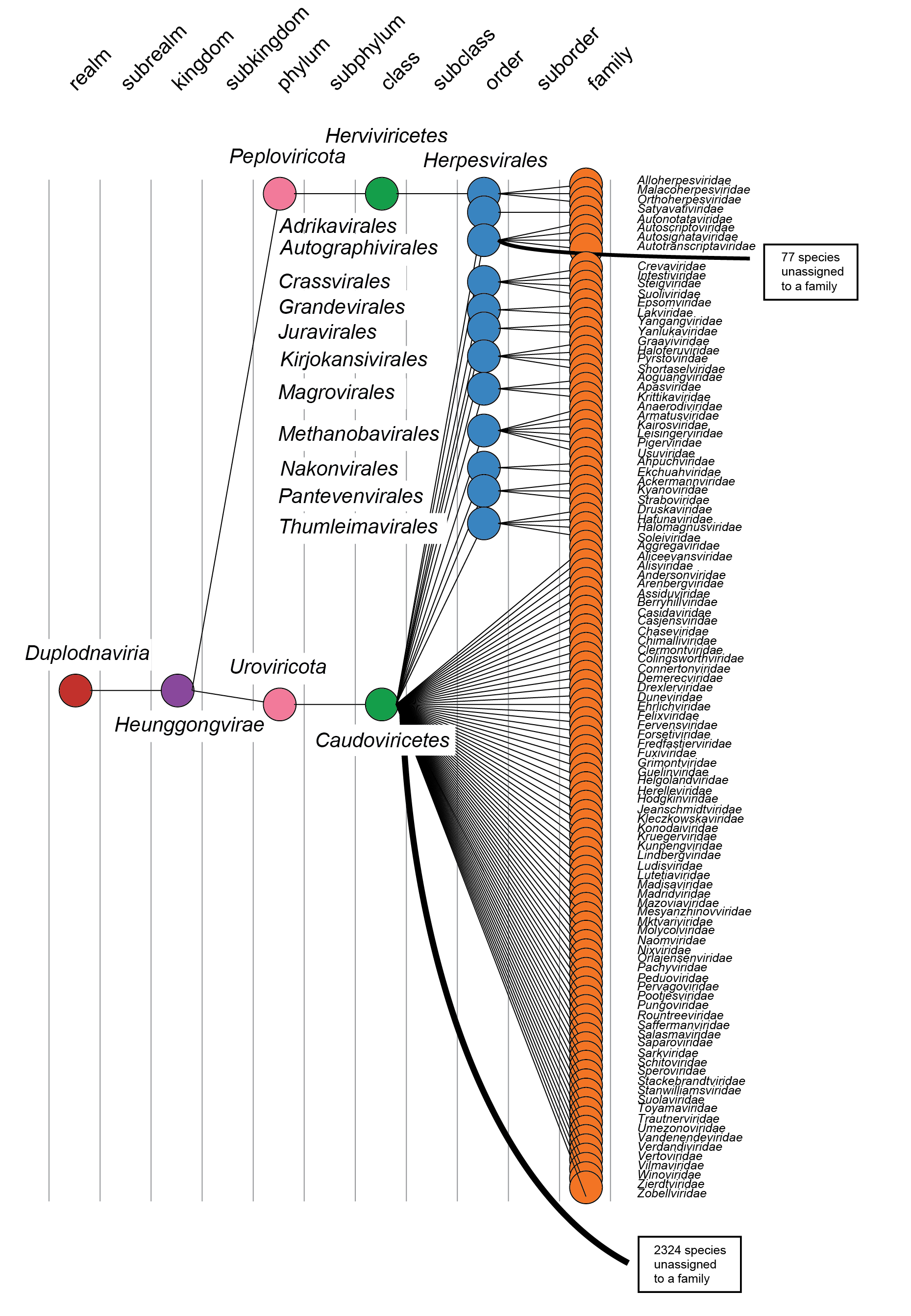 |
| Figure 5 Duplodnaviria. Taxonomy of the realm Duplodnaviria. |
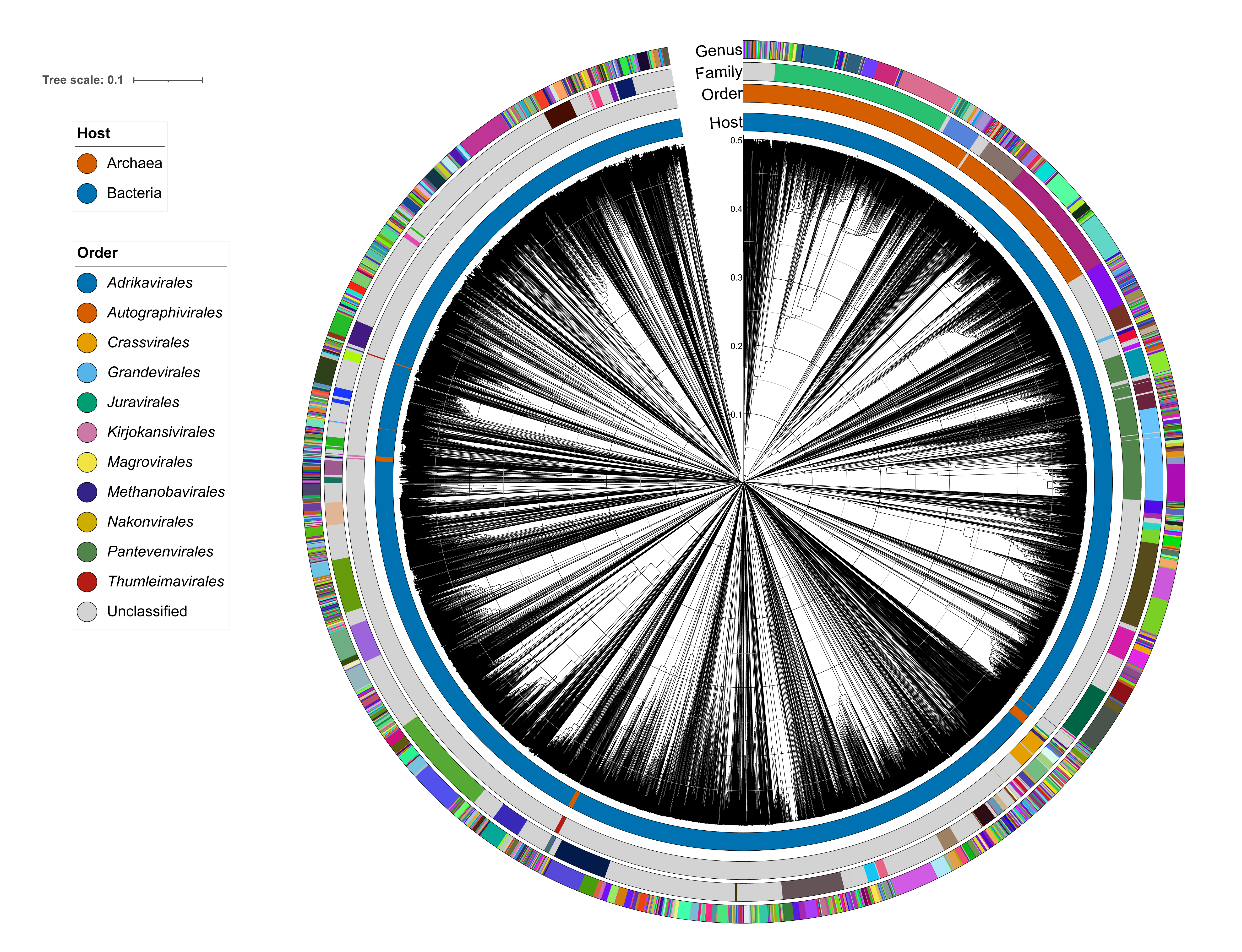 |
| Figure 6 Duplodnaviria. Virus proteomic tree of all classified caudoviricetes (MSL#40), generated with VipTree and visualised in iToL. Genome sequences were compared to each other pairwise with tBLASTx to generate a distance matrix visualised as a neighbour joining tree. Branch lengths reflect the genomic distances between two virus genomes on a scale from 0 (identical genomes) to 1 (no detectable amino acid identity). Coloured rings represent the host and taxonomy information based on VMR#40, with colours randomly assigned. |
Related, unclassified viruses
Recently, a phylogenetic guided metagenomic survey of the sunlit oceans has revealed a group of eukaryotic plankton-infecting viruses that form a putative new phylum dubbed “Mirusviricota”. The virion morphogenesis module of this large clade is typical of viruses from the realm Duplodnaviria, and includes a distinct HK97-fold MCP as well as the other hallmark genes of Duplodnaviria. These viruses have not yet been classified but are clearly related to members of the Duplodnaviria (Gaïa et al., 2023).

