Family: Adenoviridae
Genus: Atadenovirus
Distinguishing features
Atadenoviruses are serologically distinct from viruses in the other adenovirus genera, and their genomic organization and capsid protein complements also differ (Vrati et al., 1996). Atadenoviruses have been detected in a broad range of hosts, predominantly including scaled reptiles (order Squamata): snakes (Benkő et al., 2002, Garner et al., 2008, Abbas et al., 2011), lizards (Wellehan et al., 2004, Papp et al., 2009, Ball et al., 2014, Prado-Irwin et al., 2018, Hyndman et al., 2019), and worm lizards (Szirovicza et al., 2016); but also birds (Harrach et al., 1997, Hess et al., 1997, To et al., 2014, Duarte et al., 2019, Needle et al., 2019b, Athukorala et al., 2020) and ruminants (Vrati et al., 1996, Harrach et al., 1997, Dán et al., 1998, Fox et al., 2017, Miller et al., 2017, Woods et al., 2018, Paim et al., 2021). There have been sporadic reports on their occurrence in marsupials (Thomson et al., 2002, Gál et al., 2017) and non-squamate reptiles (tortoises) (Garcia-Morante et al., 2016, Salzmann et al., 2021).
Virion
Morphology
Atadenovirus particles are largely similar to those of mastadenoviruses, except for the prominent knobs formed by protein LH3 at the icosahedral and local 3-fold axes in the facet (Menéndez-Conejero et al., 2017). This distinguish atadenoviruses structurally from other known adenoviruses (Figure 1. Atadenovirus). LH3 is located in the same relative position among the hexon subunits as protein IX (present only in mastadenoviruses) but sits on top of the hexon towers, rather than in the valley between hexon bases (Liu et al., 2010, Menéndez-Conejero et al., 2017). Lizard adenovirus 2 (LAdV-2) LH3 contacts the capsid surface via a triskelion structure identical to that used by mastadenovirus protein IX (Marabini et al., 2021). The high resolution structure of LAdV-2 shows a large conformational difference in the internal vertex protein IIIa between mast- and atadenoviruses, in form of the presence of an extended polypeptide (Marabini et al., 2021). This polypeptide and α-helical clusters beneath the facet are proposed to correspond to genus-specific proteins LH2 and p32K. Ovine adenovirus 7 (OAdV-7) particles that lack the LH3 or p32K proteins are viable although less stable (Pantelic et al., 2008). Similarly, psittacine adenovirus 3 (PsAdV-3) seems to lack LH3 (To et al., 2014). (All non-mastadenoviruses lack proteins V and IX but are still viable.)
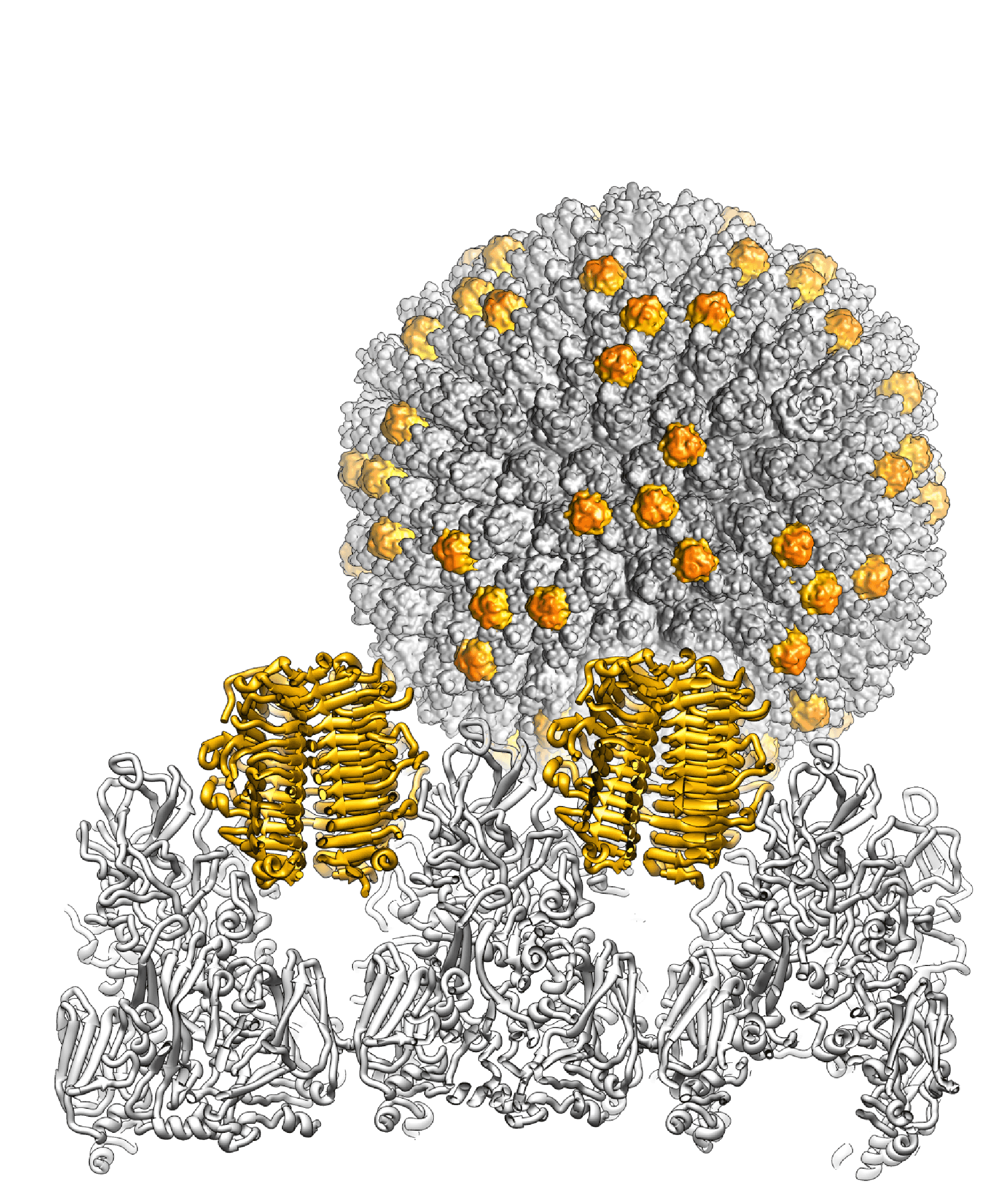 |
| Figure 1. Atadenovirus The cryo-EM map of snake adenovirus 1 virion at 11 Å resolution is shown in the background, with the genus-specific minor coat protein LH3 highlighted in yellow. In the foreground, the crystal structure of LH3 (yellow) and its position with respect to hexons (grey) is shown in a view across the particle. LH3 has a fold typical of bacteriophage tailspikes, and makes multiple contacts with hexons to stabilize the capsid (Menéndez-Conejero et al., 2017). Image courtesy of Carmen San Martin. |
LAdV-2 (from Mexican beaded lizard) has three long fibers in some vertices and single short fibers in the rest (Pénzes et al., 2014). The 3D structures of snake adenovirus 1 (SnAdV-1) (Singh et al., 2014) and bovine adenovirus 4 fibers have been established (Nguyen et al., 2015).
Physicochemical and physical properties
Virions possess elevated heat stability and retain substantial infectivity after treatment for 30 min at 56°C (Bartha and Kisary 1970). These conditions inactivate mastadenovirus particles.
Nucleic acid
The genome size of sequenced isolates ranges from 29,576 (OAdV-7) (Vrati et al., 1996) to 39,642 bp (eastern spinebill adenovirus, “passerine adenovirus 1”) (Athukorala et al., 2020) with inverted terminal repeats (ITRs) of 36 (bovine adenovirus 7) to 195 bp (bearded dragon adenovirus 1, BDAdV-1) (Dán et al., 2001, Pénzes et al., 2020b, Kumagai et al., 2021). For ruminant atadenoviruses, duck adenovirus 1 (DAdV-1) and common tern adenovirus 1, the G+C content of the DNA is low, varying between 33.24 (deer adenovirus 1 [OdAdV-1]), and 43.01% (DAdV-1). The corresponding high A+T content was deemed sufficiently characteristic to be used as the basis for naming the genus. However, it turned out that atadenoviruses originating from scaled reptiles have a non-biased nucleotide composition (50.22 G+C in SnAdV-1) (Farkas et al., 2008), 44.15% in LAdV-2 (Pénzes et al., 2014) and 56.21% in BDAdV-1). Additional newly identified avian atadenoviruses also have a non-biased nucleotide composition (52.88–53.75% G+C in white-eyed parakeet adenovirus 1 (Duarte et al., 2019), PsAdV-3 and eastern spinebill adenovirus 1 (“passerine adenovirus 1”) (Athukorala et al., 2020).
Proteins
The proteins of a typical atadenovirus are summarized in Table 1. Atadenovirus (Vrati et al., 1996). Atadenoviruses have no protein V and IX but have a unique structural protein p32K. LH2 has also been found in purified atadenovirus particles (SnAdV-1), and is therefore another genus-specific structural protein (Menéndez-Conejero et al., 2017).
Table 1. Atadenovirus. Proteins encoded by OAdV-7
|
kDa |
Transcription class |
Description |
Note |
|
32 |
Unknown |
S; p32K† |
Unique to atadenoviruses |
|
13 |
LH1 |
R |
Missing in DAdV-1 |
|
14.7 |
LH2 |
S |
|
|
42.8 |
LH3 Unknown |
R, S |
Distant homologue of mastadenovirus E1B 55K, missing in PsAdV-3 |
|
43 |
E2 |
D; DBP |
|
|
123 |
E2 |
D; DNA pol |
|
|
67.1 |
E2 |
S; pTP† |
|
|
12.9, 20.9, 19.8, 19.8 |
RH1, RH2, RH4, RH6, early |
R |
F-box proteins unique for atadenoviruses. Related to each other; missing in PsAdV-3. |
|
22.6 |
RH5, early |
R |
Missing in DAdV-1, PsAdV-3, |
|
17.1 |
E4.1, early |
R |
Missing in DAdV-1 |
|
25.6, 30.8 |
E4.2, E4.3, early |
R |
Distant homologues of mastadenovirus E4 34K |
|
38.2 |
Early and late |
D; 52/55 kDa*,† |
|
|
58.4 |
Late |
S (pIIIa);† |
|
|
51 |
Late |
S (III); penton base* |
Lacks integrin-binding motif |
|
12.9 |
Late |
S (pVII);† major core |
|
|
7.3 |
Late |
S (pX);† X/µ |
|
|
24.5 |
Late |
S (pVI)† |
|
|
102 |
Late |
S (II); hexon |
|
|
23 |
Late |
D, S; protease |
|
|
72 |
Late |
D; 100 kDa* |
Hexon assembly protein |
|
15.7 |
Late |
D, R; 33 kDa* |
p-protein not found in DAdV-1 |
|
24.7 |
Late |
S (pVIII)† |
|
|
58.2 |
Late |
S (IV); fiber |
Cell attachment protein. Fibers of two lengths in LAdV-2, PsAdV-3 and white-eyed parakeet adenovirus 1. |
|
37.5 |
Unknown |
D, S (IVa2); packaging |
|
Molecular masses are presented as unmodified and uncleaved gene products. D = DNA synthesis and packaging; DBP = DNA-binding protein; DNA pol = DNA polymerase; LH = left end [genes]; p = precursor; p-protein = phosphoprotein; R = regulation, RH = right end [genes]; S = structural; TP = terminal protein; All non-structural proteins are hypothetical until characterized. * = Mr values are significantly different from those obtained by SDS-PAGE; † = cleaved by viral protease.
Lipids
None reported.
Carbohydrates
See discussion under family properties.
Genome organization and replication
The proteins encoded by an atadenovirus genome are summarized in Table 1. Atadenovirus (Vrati et al., 1996). The central part of the atadenovirus genome is similar to that of mastadenoviruses (except that there are no protein V and IX genes), whereas the proteins encoded by the extremities of the genomes differ markedly (Vrati et al., 1996, Hess et al., 1997, Farkas et al., 2008, Pénzes et al., 2014, To et al., 2014, Miller et al., 2017, Pénzes et al., 2020b). The left end of the genome carries a gene for p32K, a unique structural protein. At this end, gene LH1 is also unique to the genus but is not present in all members (it seems to be missing in the avian atadenoviruses). LH2 is another genus-specific gene (Menéndez-Conejero et al., 2017). The right end of the genome contains genes that are related to each other, suggestive of gene duplication. There are two E4 34K gene homologs (E4.2 and E4.3), and one to six RH gene homologues. At this end of the genome, genes E4.1 and RH0–RH6 are also unique to the genus but are not present in all members. The proteins encoded by genes LH3 and E4.3 (and its paralog, E4.2) show limited similarity to mastadenovirus proteins E1B 55K and E4 34K, respectively, and (attached to each other) have been proposed to play a similar role, i.e. targeting cellular proteins for degradation (Gilson et al., 2016).
No immunomodulatory genes such as those found in the mastadenovirus E3 region have yet been identified. DAdV-1 and PsAdV-3 have a unique region at the far right end of the genome, with seven and six uncharacterized ORFs, respectively. Six and five of these are different in these two atadenoviruses and may be avian host-specific, whereas both viruses have ORF1, which is shared by LAdV-2 and BDAdV-1, both of these reptile atadenoviruses having also five further genes (not identical) in this region (Pénzes et al., 2014, Pénzes et al., 2020b). ORF4 and ORF5 of PsAdV-3 are related to each other and also to ORF2 of the aviadenoviruses psittacine adenovirus 1 (PsAdV-1) and psittacine adenovirus 4 (PsAdV-4), and ORF52 of the aviadenoviruses pigeon adenovirus 1 (PiAdV-1), and pigeon adenovirus 2. (In these two psittacine and two pigeon aviadenovirus types, these four homologous genes localize at the far left end of the genome instead of the right end). This unique region of DAdV-1 also contains a VA RNA gene that may be homologous to that of the aviadenovirus fowl adenovirus 1 (FAdV-1). The recently described BDAdV-1 genome harbours three genes in this region that encode proteins of the C-type lectin-like domain superfamily (Pénzes et al., 2020b). The protein named ORF3 in this virus has a CTLD group II-like domain architecture displaying structural similarity to natural killer cell surface receptors and with an alphaherpesvirus virulence factor gene for neurotropism, UL45.
The receptor-binding properties of DAdV-1 were predicted from the crystal structure of the fiber head to involve binding to the chicken coxsackievirus and adenovirus receptor (CAR) (Song et al., 2019). Splicing is presumed in the IVa2, pTP and 33K genes but not in the DNA polymerase gene of atadenoviruses except BDAdV-1 (Pénzes et al., 2020b).
Biology
Certain atadenoviruses can cause haemorrhagic epizootic disease in free-living ruminants (Fox et al., 2017, Miller et al., 2017, Woods et al., 2018). DAdV-1 is also associated with a specific disease of hens that is characterized globally by sharp decreases in egg production (egg drop syndrome) and egg shell deformations (Hess et al., 1997). BDAdV-1 has been linked to sudden death, lethargy, weakness, diarrhoea, dehydration and anorexia, and it is responsible for central nervous system signs observed in young bearded dragons (“star gazing”, head tilt, opisthotonos, paresis and circling) (Pénzes et al., 2020b).
Due to the lack of pre-existing immunity in humans and its low bio-safety profile, OAdV-7 has been developed as a gene delivery vector intended for human vaccine and gene therapy applications (Both 2004).
Antigenicity
See discussion under family properties.
Species demarcation criteria
Species designation depends on at least two of the following characteristics:
- Phylogenetic distance (>10–15%, based on distance matrix analysis of the DNA polymerase amino acid sequence)
- Host range
- Nucleotide composition
- Cross-neutralization
- Gene organization at the right end of the genome
Member species
The Member Species table enumerating important virus exemplars classified under each species of the genus is provided at the bottom of the page.
Related, unclassified viruses
|
Virus name |
Accession number |
Virus abbreviation |
|
amphisbaenian adenovirus 1 |
|
|
|
anolis adenovirus 1 |
|
|
|
anolis adenovirus 2 |
|
|
|
anolis adenovirus 3 |
|
|
|
blue-tongued skink adenovirus |
|
|
|
bovine adenovirus 7 |
BAdV-7 |
|
|
chameleon adenovirus 1 |
ChAdV-1 |
|
|
chameleon adenovirus 2 |
ChAdV-2 |
|
|
chimney swift adenovirus 1 |
CSAdV-1 |
|
|
common chaffinch adenovirus |
|
|
|
common tern adenovirus 1 |
|
|
|
eastern spinebill adenovirus 1 (passerine adenovirus 1) |
|
|
|
emerald monitor adenovirus |
|
|
|
Eurasian bullfinch adenovirus |
|
|
|
Eurasian siskin adenovirus |
|
|
|
European greenfinch adenovirus strain 47975 |
|
|
|
European robin adenovirus |
|
|
|
gecko adenovirus 1 |
GeAdV-1 |
|
|
Greek (spur-thighed) tortoise adenovirus 1 |
|
|
|
Japalura tree dragon adenovirus 1 |
|
|
|
lacertid adenovirus 1 |
|
|
|
lacertid adenovirus 2 |
|
|
|
lizard adenovirus 1 |
LAdV-1 |
|
|
long-tailed grass lizard adenovirus 1 |
|
|
|
Russian (Horsfield’s) tortoise adenovirus |
|
|
|
snake adenovirus 2 |
SnAdV-2 |
|
|
snake adenovirus 3 |
SnAdV-3 |
|
|
song thrush adenovirus |
|
|
|
tokay gecko adenovirus |
|
|
|
tropical screech owl adenovirus 1 |
|
|
|
vitelline masked weaver adenovirus strain 37869 |
|
|
|
vitelline masked weaver adenovirus strain 38132 |
|
|
|
white-eyed parakeet adenovirus 1 |
|
|
|
white plumed honeyeater adenovirus 1 |
|
|
|
white plumed honeyeater adenovirus 2 |
|
|
|
white-throated monitor adenovirus 1 |
|
Virus names and virus abbreviations, are not official ICTV designations.
Not all adenovirus names have generally accepted and applied abbreviations.

