Family: Mymonaviridae
Dàohóng Jiāng (姜道宏), María A. Ayllón, Shin-Yi L. Marzano, Hideki Kondō (近藤秀樹) and Massimo Turina
The citation for this ICTV Report chapter is the summary published as Jiāng et al., (2022):
ICTV Virus Taxonomy Profile: Mymonaviridae 2022, Journal of General Virology, (2022) 103:001787
Corresponding author: Dàohóng Jiāng (姜道宏) (daohongjiang@mail.hzau.edu.cn)
Edited by: Jens H. Kuhn and Sead Sabanadzovic
Posted: June 2019, August 2022, May 2023
PDF: ICTV_Mymonaviridae.pdf (2019 version)
Summary
Typical members of the family Mymonaviridae produce filamentous, enveloped virions containing a single molecule of linear, negative-sense RNA of about 10 kb; whether other members produce virions is unknown (Table 1.Mymonaviridae). The family includes nine genera, some of which include multiple species. Mymonavirids usually infect filamentous fungi, although they have also been identified in metagenomics studies of insects, oomycetes, and plants. At least one virus, Sclerotinia sclerotiorum negative-stranded RNA virus 1, induces hypovirulence in its fungal host.
Table 1. Mymonaviridae. Characteristics of members of the family Mymonaviridae
| Characteristic | Description |
| Example | Sclerotinia sclerotiorum negative-stranded RNA virus 1 (KJ186782), species Sclerotimonavirus sclerotinae, genus Sclerotimonavirus |
| Virion | Enveloped, filamentous virions 25–50 nm in diameter and about 1,000 nm in length |
| Genome | Single molecule of linear, negative-sense RNA of about 10 kb |
| Replication | Ribonucleoprotein (RNP) complexes containing anti-genomic RNA serve as templates for synthesis of nascent RNP complexes containing genomic RNA |
| Translation | The virus RNA-directed RNA polymerase binds the encapsidated genome at the leader region and then sequentially transcribes each gene by recognizing start and stop signals flanking viral genes. This produces subgenomic RNAs that serve as mRNAs |
| Host range | Fungi; also detected in metagenomics studies of insects, oomycetes, plants, and soil |
| Taxonomy | Realm Riboviria, phylum Negarnaviricota, subphylum Haploviricotina, class Monjiviricetes, order Mononegavirales; the family Mymonaviridae includes nine genera (Auricularimonavirus, Botrytimonavirus, Hubramonavius, Lentimonavirus, Penicillimonavirus, Phyllomonavirus, Plasmopamonavirus, Rhizomonavirus and Sclerotimonavirus) and 50 species |
Virion
Morphology
Virions of a typical member, Sclerotinia sclerotiorum negative-stranded RNA virus 1 (SsNSRV-1), are filamentous, 25–50 nm in diameter, about 1,000 nm in length and enveloped by a membrane (Figure 1A.Mymonaviridae). The outer surface of virions does not appear to be covered with spikes. Nucleocapsids released from virions are single, left-handed, helical structures that, when tightly coiled, have a diameter of 20–22nm and a length of 200–2,000 nm (Figure 1B.Mymonaviridae). Nucleocapsids consist of polymerized nucleoprotein (NP) monomers (Figure 1C.Mymonaviridae) (Liu et al., 2014). Whether other members produce virions is unknown.
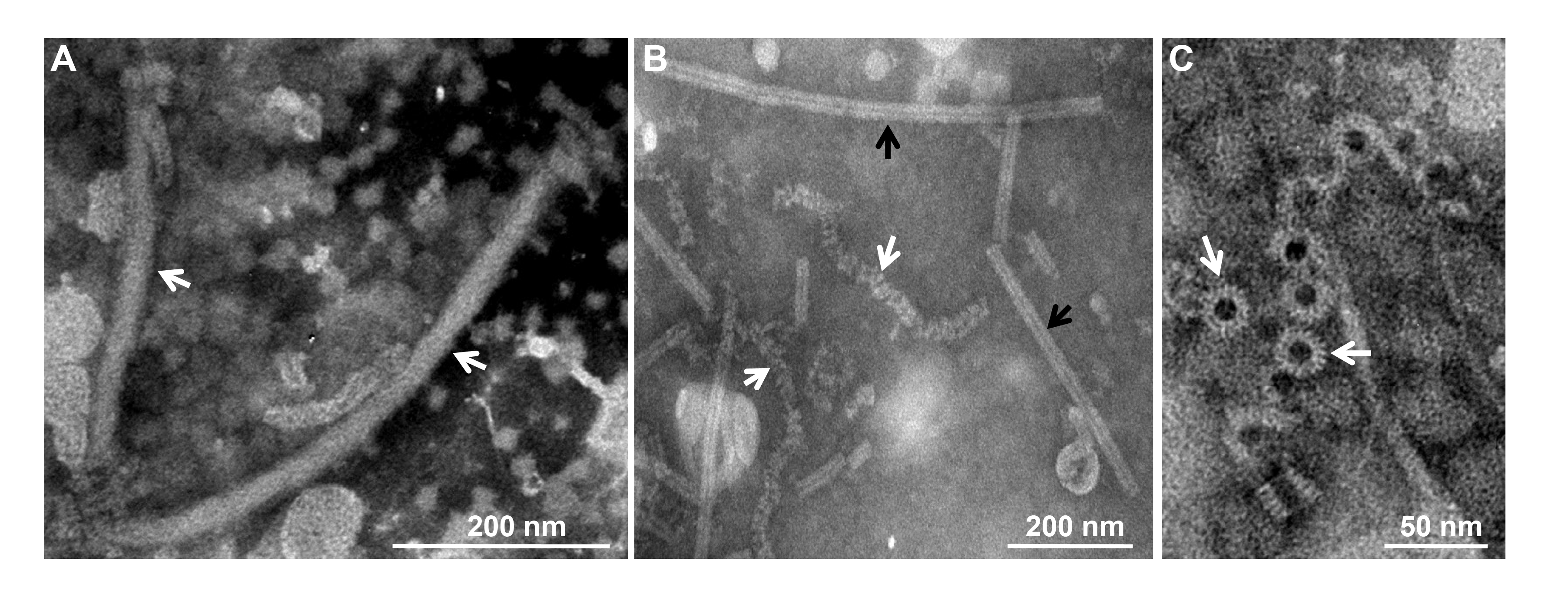 |
| Figure 1. Mymonaviridae. Morphology and structure of virions and ribonucleoprotein (RNP) complexes. (A) Filamentous, enveloped virions (marked by white arrows) and RNP complexes. (B) Purified tight (black arrows) or loose coils (white arrows) of RNP complexes. (C) Rings (white arrows) constitute the coils and nucleoprotein (NP) monomers (modified from (Liu et al., 2014)).s. |
Physicochemical and physical properties
Not reported.
Nucleic acid
A typical mymonavirion contains a single molecule of a linear, negative-sense RNA nucleic acid of about 10 kb (Figure 2.Mymonaviridae, Table 2.Mymonaviridae). The genome of SsNSRV-1 lacks a poly(A) tail at the 3′-terminus and is uncapped at the 5′-terminus. The two termini are not complementary in sequence. Defective viral RNA genomes with incomplete 5′- and 3′-termini can be found in virion preparations (Liu et al., 2014).
Table 2. Mymonaviridae. Mymonavirus isolate details (still to be updated)
Species Virus isolate | Accession number | Available sequence (nt) | Virus Abbreviation |
Dadou sclerotimonavirus soybean leaf-associated negative-stranded RNA virus 3-1* |
|
6,218 |
SLaNSRV-3 |
Drop sclerotimonavirus Sclerotinia sclerotiorum negative-stranded RNA virus 2-500 Sclerotinia sclerotiorum negative-stranded RNA virus 4-257 |
|
9,608 9,707 |
SsNSRV-2 SsNSRV-4 |
Glycine sclerotimonavirus Fusarium graminearum negative-stranded RNA virus 1-HN1 soybean leaf-associated negative-stranded RNA virus 1-1# |
|
9,072 9,041 |
FgNSRV-1 SLaNSRV-1 |
Hubei sclerotimonavirus Hubei rhabdo-like virus 4-arthropodmix 13990 |
|
10,003 |
HbRLV-4 |
Illinois sclerotimonavirus soybean leaf-associated negative-stranded RNA virus 2-1* |
|
7,321 |
SLaNSRV-2 |
Phyllosphere sclerotimonavirus soybean leaf-associated negative-stranded RNA virus 4-1* |
|
5,317 |
SLaNSRV-4 |
Sclerotinia sclerotimonavirus Sclerotinia sclerotiorum negative-stranded RNA virus 1-AH98 Sclerotinia sclerotiorum negative-stranded RNA virus 3-IL1 |
|
10,002 10,009 |
SsNSRV-1 SsNSRV-3 |
*Coding region sequence complete, but genome incomplete
# Coding region sequence incomplete.
Proteins
Mymonavirids express one or more proteins (Figure 2.Mymonaviridae). SsNSRV-1 expresses at least six proteins, the nucleocapsid possibly contains two nucleoproteins (NP) with different molecular masses, about 43 kDa and about 41 kDa, or protein p41 may be a degraded product of p43. The nucleoproteins encapsidate the virus genome. The RdRP, a domain of the large protein (L), mediates virus genome replication and transcription. The functions of the remaining four proteins are unclear.
Lipids
Not reported.
Carbohydrates
Not reported.
Genome organization and replication
The SsNSRV-1 genome has six major non-overlapping open reading frames (encoding proteins, p I, NP, p III, p IV, L protein and p VI (Figure 2.Mymonaviridae). These ORFs are separated by non-coding intergenic regions containing highly conserved gene-junction sequences (Figure 3.Mymonaviridae) and are expressed as individual transcription units (Figure 4.Mymonaviridae). ORFs II–VI are located in the +2 reading frame, whereas ORF I is located in the +1 reading frame. ORF II encodes NP, and ORF V encodes L protein. Other mymonavirus genomes contain four to seven ORFs. The exact size and the number of ORFs of viruses whose genomes are incompletely sequenced are unknown; penicilimonaviruses have ORFs on both strands, in which N protein is encoded on the positive-sense strand (Figure 2.Mymonaviridae).
Mymonavirids are believed to replicate in the cytoplasm of host cells, but their replication strategies are not well studied. RNP complexes can be used directly as templates for replication and transcription. Replication usually occurs within RNP complexes and requires L protein to synthesize full-length positive-sense antigenomes that later serve as templates for the synthesis of negative-sense progeny genomes.
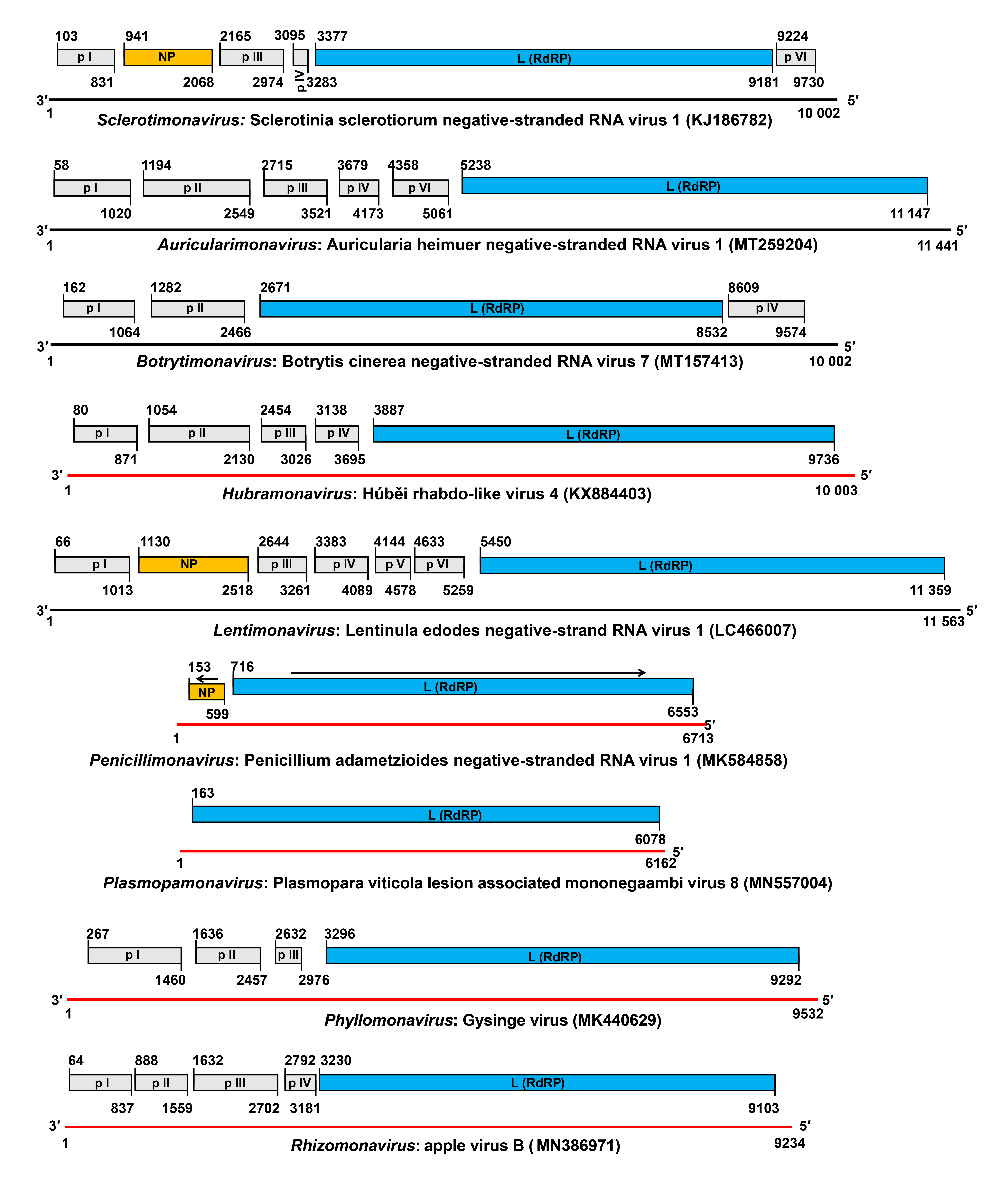 |
| Figure 2. Mymonaviridae. Genome organization of each genus. Boxes indicate position and length of each ORF and their encoded proteins, proteins are labelled with Roman numerals, except nucleoprotein (NP) and L protein including an RNA-directed RNA polymerase domain. Red lines indicate incompletely sequenced genomes. |
 |
| Figure 3.Mymonaviridae. Alignment of the putative gene-junction sequences between ORFs in a 3′→5′ orientation of SsNSRV-1. Conserved sequences [3′-(A/U)(U/A/C)UAUU(U/A)AA(U/G)AAAACUUAGG(A/U)(G/U)-5′] are highlighted in blue. Different shades indicate levels of conservation with the darkest colour indicating the highest conservation (modified from (Liu et al., 2014)) |
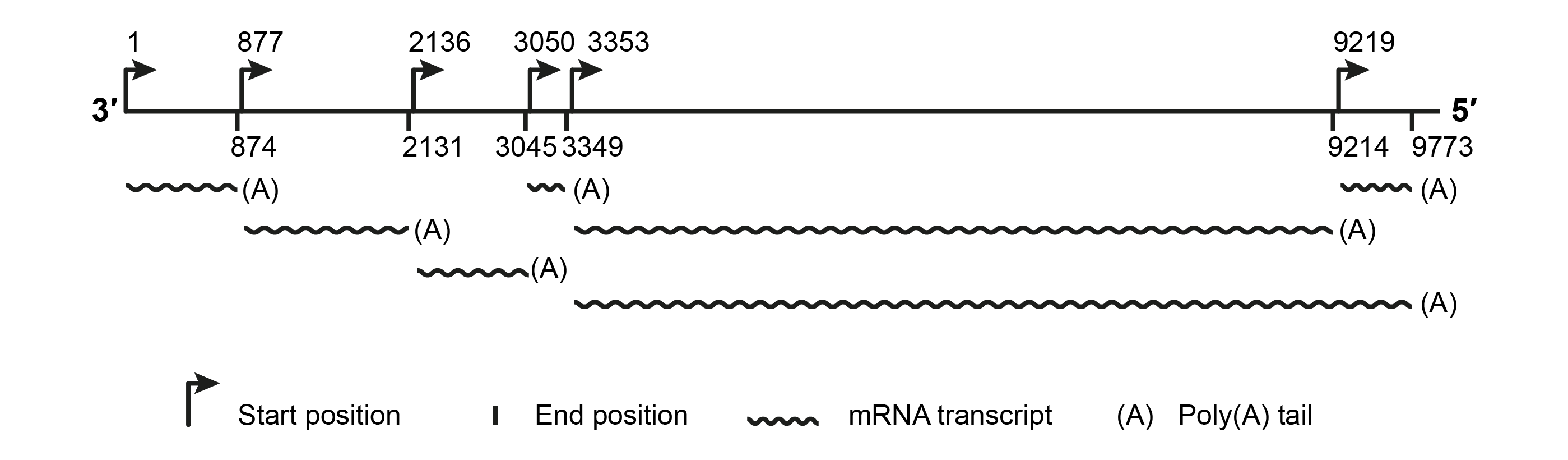 |
| Figure 4.Mymonaviridae. Deduced transcription map based on 5′- and 3′-RACE results for SsNSRV-1 (modified from (Liu et al., 2014)). |
Biology
Mymonavirids have been characterized in fungi belonging to 12 different species including plant-pathogenic, edible, and endophytic fungi (Kondo et al., 2013, Liu et al., 2014, Marzano et al., 2016, Mu et al., 2017, Hao et al., 2018, Wang et al., 2018, Lin et al., 2019, Nerva et al., 2019, Ruiz-Padilla et al., 2021); they also have been found in high-throughput sequencing-generated sequences from RNA preparations from insects (Shi et al., 2016, Medd et al., 2018, Pettersson et al., 2019), oomycetes (Chiapello et al., 2020), plants (Marzano and Domier 2016, Wright et al., 2020), and soil (Starr et al., 2019), but their real hosts are unclear (Table 2.Mymonaviridae).
SsNSRV-1 was isolated from the Chinese strain AH98 of stem rot fungus (Sclerotinia sclerotiorum (Lib.) de Bary (1884)), and causes hypovirulence in that fungus (Liu et al., 2014). SsNSRV-1 infection causes its host to grow slowly, lose the ability to produce sclerotia, and lose pathogenicity on plants, such as rapeseed (Brassica napus L.). The virus can be transmitted horizontally through hyphal fusion. Transmission through sexual spores (ascospores) and asexual spores (conidia) are not known to occur.
Derivation of names
Auricularimonavirus: from Auricularia (the genus of a fungal host), and Mononegavirales.
Botrytimonavirus: from Botrytis (the genus of a fungal host), and Mononegavirales.
Hubramonavirus: from Húběi (a province of China) and rhabdo-like virus 4 (the first name given to the first virus to be described of this genus), and Mononegavirales.
Lentimonavirus: from Lentinula (the genus of a fungal host), and Mononegavirales.
Mymonaviridae: from Myco and Mononegavirales.
Penicillimonavirus: from Penicillium (the genus of a fungal host), and Mononegavirales.
Phyllomonavirus: from phyllosphere and Mononegavirales.
Plasmopamonavirus: from Plasmopara (the genus of an oomycete that may be the host of a member virus), and Mononegavirales.
Rhizomonavirus: from rhizosphere, and Mononegavirales.
Sclerotimonavirus: from Sclerotinia (the genus of a fungal host), and Mononegavirales.
Genus demarcation criteria
32% L protein amino acid sequence identity is a genus rank demarcation threshold; members of different genera are less than 32% identical.
Relationships within the family
Phylogenetic relationships across the family have been deduced from maximum-likelihood trees generated from the amino acid sequences of the RdRP core domain of mymonavirids and other selected viruses (Figure 5.Mymonaviridae). Currently, nine genera have been established in the family: Auricularimonavirus, Botrytimonavirus, Hubramonavirus, Lentimonavirus, Penicillimonavirus, Phyllomonavirus, Plasmopamonavirus, Rhizomonavirus and Sclerotimonavirus. These genera currently include a total of 37 species (Figure 5.Mymonaviridae).
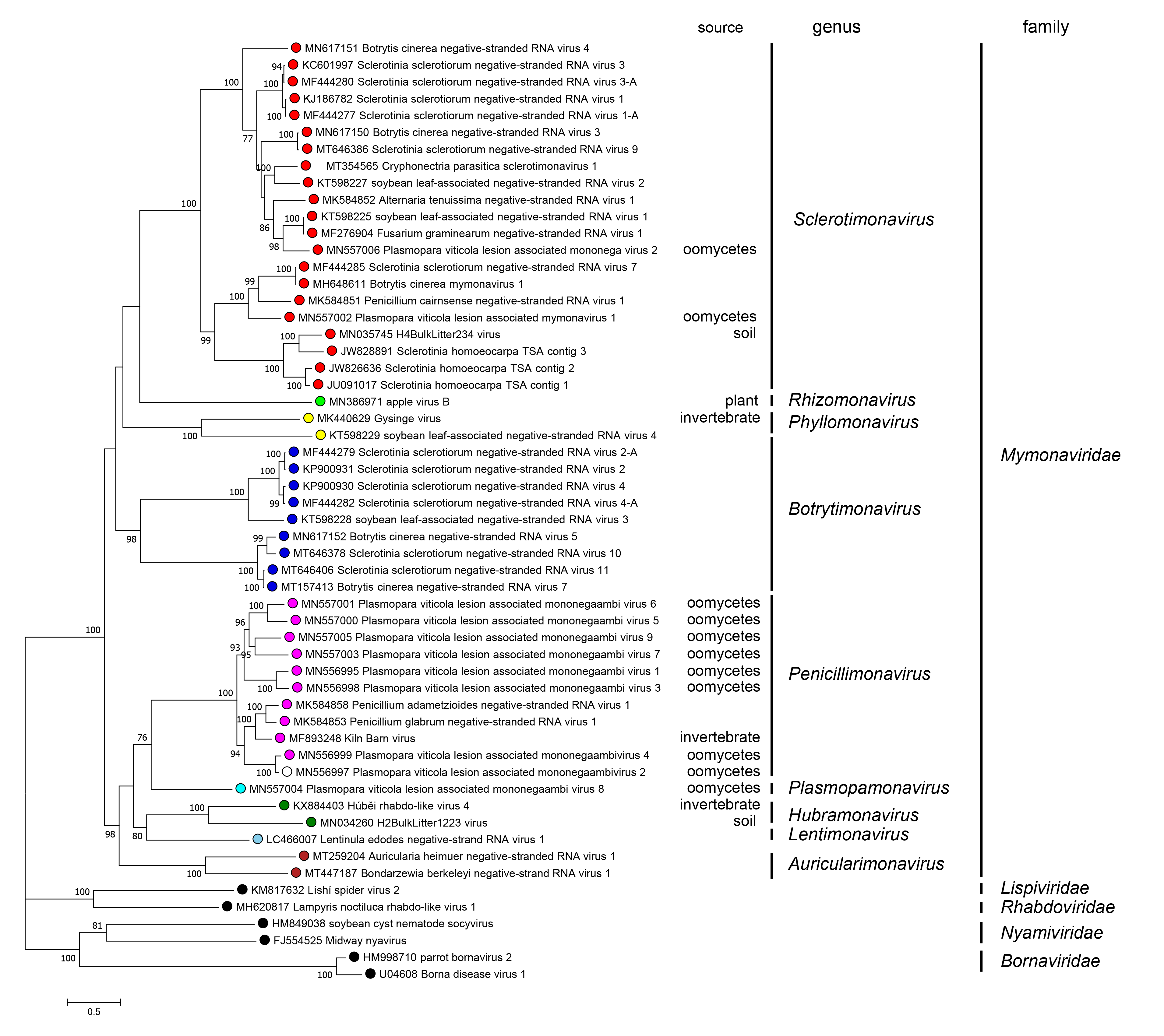 |
| Figure 5. Mymonaviridae. Phylogenetic tree of mymonavirids. A maximum likelihood phylogenetic tree was constructed based on the multiple amino acid sequence alignment of the RNA-directed RNA polymerase (RdRP) using IQ-TREE (version 1.6.11) with the best-fit model “LG+F+I+G” (Nguyen et al., 2015). The tree was midpoint-rooted for clarity of presentation and 1,000 bootstrap replicates were performed; values over 70% are indicated. Viruses classified in the families Lispiviridae, Rhabdoviridae, Nyamiviridae, and Bornaviridae were used as outgroups. The "source" column indicates the origin of the sequence if not fungal. This phylogenetic tree and corresponding sequence alignment are available to download from the Resources page. |
Relationships with other taxa
Mymonavirids form a family in the haploviricotine order Mononegavirales. Within this order, mymonavirids are most closely related to members of the families Bornaviridae, Lispiviridae, Nyamiviridae, and Rhabdoviridae.

